THE QUANTUM-CHEMICAL CRITERIA FOR INTERMOLECULAR INTERACTIONS EFFICIENCY IN COMPLEX SYSTEMS “METHANE/CARBON DIOXIDE – AROMATIC HYDROCARBONS” OF FOSSIL COAL
DOI:
https://doi.org/10.15421/jchemtech.v33i3.332701Keywords:
ab initio calculations, atoms-in-molecules theory, electron density, bond critical point, resonant vibrational frequencyAbstract
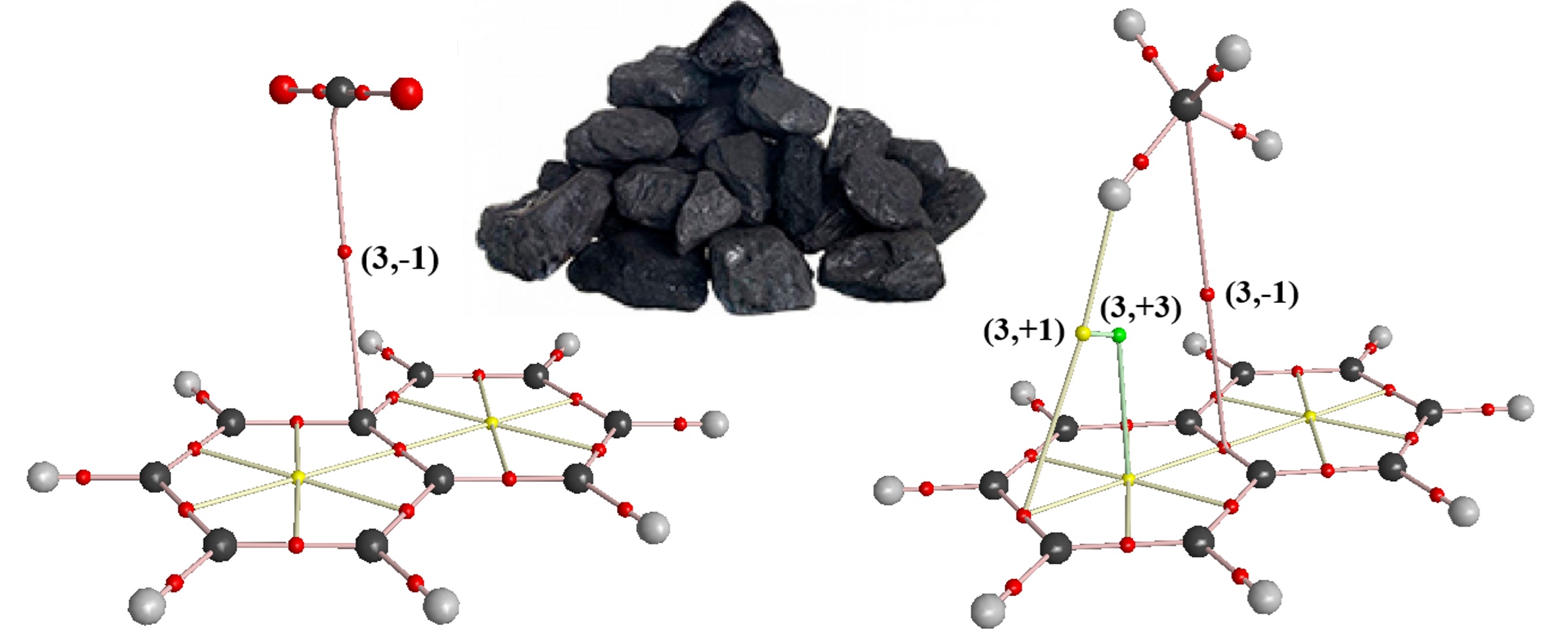
Using ab initio density functional methods, the features of intermolecular interactions that arise in complex systems based on methane and carbon dioxide with the participation of model aromatic hydrocarbons that are the part of the carbonized fossil organic matter have been investigated. A detailed topological analysis of these systems within the framework of the QT-AIM theory of R. Bader on the example of molecular complexes CH4 × C6H6, CH4 × C10H8, CH4 × 2C10H8, and also CO2 × C10H8 demonstrated the presence of (3,–1) bond critical points that arise at distances of about 3.089–3.533 Å, which can be fully characterized as weak intermolecular interactions. At the same time, as criteria for the effectiveness of such binding, it is convenient to use the parameters of the electron density r(r) as well as the Laplacian of the electron density Ñ2r(r), which in the case of the studied structures do not exceed 0.0027–0.0036 e/Å3 and 0.0022–0.0034 e/Å5 with a total stabilization energy of no more than 2.9–7.5 kJ/mol. A comparative analysis of the calculated thermodynamic characteristics of these systems under conditions of varying temperature and pressure parameters indicates a minor contribution of the enthalpy factor with predominance of the entropic component, mainly due to changes in the translational, rotational, and vibrational degrees of freedom of individual molecules. Some corresponding values of resonant vibrational frequencies are ~3162–3170 cm–1. The results of calculations are in good agreement with that data, which have been obtained for the related systems, indicating their adequate reproduction within the limits of the chosen level of theory.
References
Sudharsana, C., Anvarsha, N., Kalyani, P. (2024). Carbon-Based Nanocomposites: A Comprehensive Review of Their Multifunctional Applications. In: Parvulescu, V., Anghel, E. M. (eds.) Nanocomposites – Properties, Preparations and Applications. Nanotechnology and Nanomaterials, Intech Open. doi: 10.5772/intechopen.114402
Chen, T.-W., Kalimuthu, P., Veerakumar, P., Lin, K.-C., Chen, S.-M., Ramachandran, R., Mariyappan, V., Chitra, S. (2022). Recent Developments in Carbon-Based Nanocomposites for Fuel Cell Applications: A Review. Molecules, 27(3), 761. doi: 10.3390/molecules27030761
Tokar, A. V. (2023). [Quantum-Chemical Understanding of the IR Absorption Characteristics for Some Structural Fragments of Lignin Macromolecules]. J. Chem. Technol., 31(3), 443–450. (in Ukrainian). doi: 10.15421/jchemtech.v31i3.286083
Nawaz, F., Ali, M., Ahmad, Sh., Yong, Y., Rahman, S., Naseem, M., Hussain, S., Razzaq, A., Khan, A., Ali, F., Al Balushi, R. A., Al-Hinaai, M. M., Ali, N. (2024). Carbon based nanocomposites, surface functionalization as a promising material for VOCs (volatile organic compounds) treatment. Chemosphere, 364, 143014. doi: 10.1016/j.chemosphere.2024.143014
Shin, M., Lim, J., Park, Y., Lee, J.-Y., Yoon, J., Choi, J.-W. (2024). Carbon-based nanocomposites for biomedical applications. RSC Adv., 14(10), 7142–7156. doi: 10.1039/d3ra08946k
Wang, C., Xing, Y., Shi, K., Wang, S., Xia, Y., Li, J., Gui, X. (2024). Chemical Structure Characteristics and Model Construction of Coal with Three Kinds of Coalification Degrees. ACS Omega, 9(1), 1881–1893. doi: 10.1021/acsomega.3c08574
Liu, H.-D., Zhang, H., Wang, J.-P., Dou, J.-X., Guo, R., Li, G.-Y., Liang, Y.-H., Yu, J.-l. (2024). Construction of macromolecular model of coal based on deep learning algorithm. Energy, 294, 130856. doi: 10.1016/j.energy.2024.130856
Hata, Y., Hayashizaki, H., Takanohashi, T., Takahashi, T., Kanehashi, K., Norinaga, K. (2022). Modeling the Chemical Structures of Coals with Different Classifications Using Mean Molecular Weights. ISIJ Int., 62(5). 948–956. doi: 10.2355/isijinternational.ISIJINT-2021-459
Jia, J., Yang, Q., Liu, B., Wang D. (2023). Structural characterization and macromolecular structure construction of non-caking coal in Chicheng Mine. Sci. Rep., 13, 16931. doi: 10.1038/s41598-023-44045-2
Wu, J., Li, Z., Huang, S., Ding, C. (2025). Construction of molecular structure model of bituminous coal and study on adsorption characteristics of C2H4 and C2H2. Sci. Rep., 15, 1500. doi: 10.1038/s41598-025-85629-4
Bulat, A., Bogdanov, V., Trachevsky, V., Burchak, O., Serikov, Y. (2021). [Research of mechanisms and driving forces of the self-organization of the matrices of natural solid hydrocarbons]. Reports of the National Academy of Sciences of Ukraine, (3), 26–32 (in Ukrainian). doi: 10.15407/dopovidi2021.03.026
Frisch, M. J., Trucks, G. W., Schlegel, H. B., Scuseria, G. E., Robb, M. A., Cheeseman, J. R., Montgomery, Jr., J. A., Vreven, T., Kudin, K. N., Burant, J. C., Millam, J. M., Iyengar, S. S., Tomasi, J., Barone, V., Mennucci, B., Cossi, M., Scalmani, G., Rega, N., Petersson, G. A., Nakatsuji, H., Hada, M., Ehara, M., Toyota, K., Fukuda, R., Hasegawa, J., Ishida, M., Nakajima, T., Honda, Y., Kitao, O., Nakai, H., Klene, M., Li, X., Knox, J. E., Hratchian, H. P., Cross, J. B., Bakken, V., Adamo, C., Jaramillo, J., Gomperts, R., Stratmann, R. E., Yazyev, O., Austin, A. J., Cammi, R., Pomelli, C., Ochterski, J. W., Ayala, P. Y., Morokuma, K., Voth, G. A., Salvador, P., Dannenberg, J. J., Zakrzewski, V. G., Dapprich, S., Daniels, A. D., Strain, M. C., Farkas, O., Malick, D. K., Rabuck, A. D., Raghavachari, K., Foresman, J. B., Ortiz, J. V., Cui, Q., Baboul, A. G., Clifford, S., Cioslowski, J., Stefanov, B. B., Liu, G., Liashenko, A., Piskorz, P., Komaromi, I., Martin, R. L., Fox, D. J., Keith, T., Al-Laham, M. A., Peng, C. Y., Nanayakkara, A., Challacombe, M., Gill, P. M. W., Johnson, B., Chen, W., Wong, M. W., Gonzalez, C., Pople, J. A. (2004). Gaussian 03 (Revision E.01). Gaussian Inc., Wallingford CT.
Sordo, J. A. (2001). On the use of the Boys-Bernardi function counterpoise procedure to correct barrier heights for basis set superposition error. J. Mol. Struct. THEOCHEM, 537(1–3), 245–251. doi: 10.1016/S0166-1280(00)00681-3
Merrick, J. P., Moran, D., Radom, L. (2007). An Evaluation of Harmonic Vibrational Frequency Scale Factors. J. Phys. Chem. A., 111(45), 11683–11700. doi:
1021/jp073974n
Mazumdar, P., Choudhury, D. (2022). Study of the alkyl-π interaction between methane and few substituted pyrimidine systems using DFT, AIM and NBO calculations. Comput. Theor. Chem., 1208, 113560. doi: 10.1016/j.comptc.2021.113560
Smith, D. G., Patkowski, K. (2013). Interactions between Methane and Polycyclic Aromatic Hydrocarbons: A High Accuracy Benchmark Study. J. Chem. Theory Comput., 9(1), 370–389. doi: 10.1021/ct3008809
Munshi, P., Guru Row, T. N. (2005). Charge density based classification of intermolecular interactions in molecular crystals. Cryst Eng Comm., 7(100), 608–611. doi: 10.1039/B511944H
Zhikol, O. A., Shishkin, O. V., Lyssenko, K. A., Leszczynski, J. (2005). Electron density distribution in stacked benzene dimers: A new approach towards the estimation of stacking interaction energies. J. Chem. Phys., 122(14), 144104. doi: 10.1063/1.1877092
Hill, J. G., Platts, J. A., Werner, H.-J. (2006). Calculation of intermolecular interactions in the benzene dimer using coupled-cluster and local electron correlation methods. Chem. Phys. Phys. Chem., 8(35), 4072–4078. doi: 10.1039/b608623c
Bulat, A., Burchak, O., Trachevskyi, V., Tokar, A. (2023). Evolution of Electron Structure of the Methane-Coal Sorption System Components and Properties. In: Guz, A. N., Altenbach, H., Bogdanov, V., Nazarenko, V. M. (eds) Advances in Mechanics. Current Research Results of the NAS of Ukraine. Advanced Structured Materials, 191, 91–101. doi: 10.1007/978-3-031-37313-8_5
Kolandaivel, P., Nirmala, V. (2004). Study of proper and improper hydrogen bonding using Bader’s atoms in molecules (AIM) theory and NBO analysis. J. Mol. Struct., 694(1–3), 33–38. doi: 10.1016/j.molstruc.2004.01.030
Kumar, P. S. V., Raghavendra, V., Subramanian, V. (2016). Bader’s Theory of Atoms in Molecules (AIM) and its Applications to Chemical Bonding. J. Chem. Sci., 128, 1527–1536. doi: 10.1007/s12039-016-1172-3
Williams, A., Williams, J. (2003). Free Energy Relationships in Organic and Bio-Organic Chemistry. Cambridge, UK: Royal Society of Chemistry.
Anslyn, E. V., Dougherty, D. A. (2005). Modern Physical Organic Chemistry. Sausalito, USA: University Science.
Benson, S. W. (1976). Thermochemical Kinetics. John Wiley & Sons, New York-London-Sydney-Toronto. doi: 10.1002/bbpc.19770810919
Bezruchko, K. A., Pymonenko, L. I., Burchak, A. V., Suvorov, D. A. (2018). Transformation of the energy state of the molecular structure of coal in the process of metamorphism. Journal of Geology, Geography and Geoecology, 27(1), 30–34. doi: 10.15421/111827
Tokar, A., Chihvintseva, O., Mirjanić, D. (2024). The Quantum-Chemical Aspects of Structuring for Some Aramide-Type Polymer Systems with Hetaryl Fragments. In: Karabegovic, I., Kovačević, A., Mandzuka, S. (eds.) New Technologies, Development and Application VII. NT 2024. Lecture Notes in Networks and Systems, 1070, 589–596. doi: 10.1007/978-3-031-66271-3_63
Tokar, A., Chigvintseva, O. (2021). The quantum-chemical and spectral criteria for hydrogen bonding efficiency in structural analysis of aramides. Chem. Chem. Technol., 15(1), 9–15. doi: 10.23939/chcht15.01.009
Downloads
Published
Issue
Section
License
Copyright (c) 2025 Oles Honchar Dnipro National University

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

