POLYELECTROLYTE FILMS BASED ON SODIUM ALGINATE AND CHITOSAN: PREPARATION, PHYSICOCHEMICAL PROPERTIES, KINETICS OF CALCIUM PANTOTHENATE RELEASE
DOI:
https://doi.org/10.15421/jchemtech.v33i4.332779Keywords:
physicochemical properties of polyelectrolyte films, surface morphology, alginate, chitosan, calcium pantothenate, polyelectrolyte complexes, kinetics of prolonged drug releaseAbstract
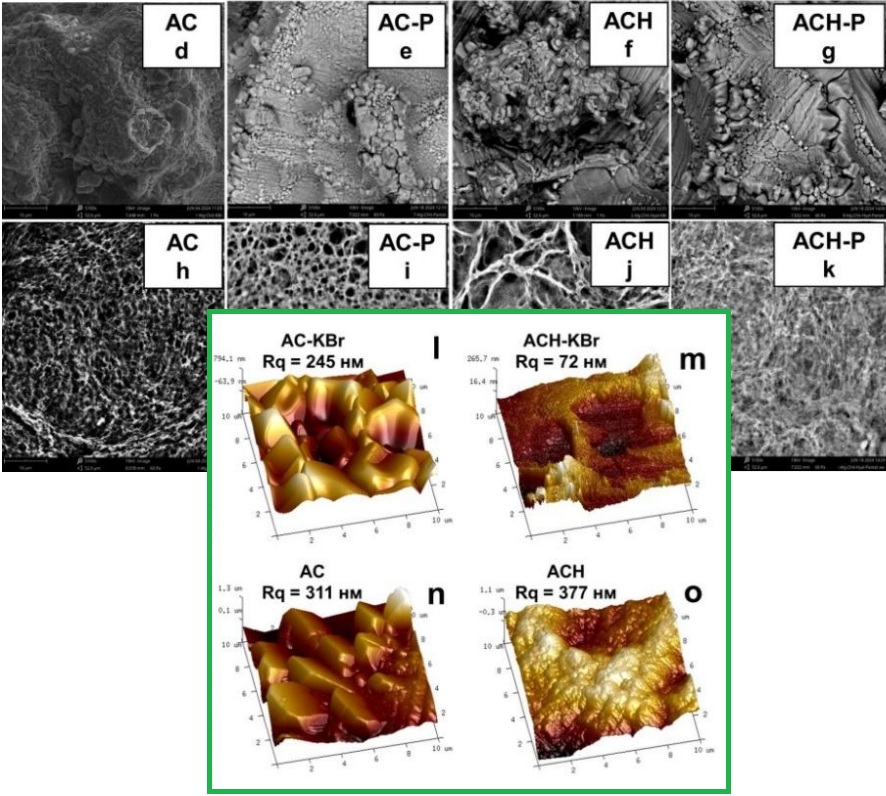
A forming method based on alginate-chitosan polyelectrolyte complexes was developed, and the physicochemical properties of the resulting films were investigated. Ionic-strength-induced suppression of electrostatic interactions was employed to control complex precipitation during assembly. Films based on alginate-chitosan complex (AC) and its sodium hyaluronate modified version (AСН) were successfully obtained. The morphology of the obtained films was investigated by scanning electron microscopy, atomic force microscopy, and low-temperature nitrogen sorption-desorption. It was established that the films have a porous structure (pore surface area is from 2 to 5 m2/g, pore volume 5–10·10-3 cm3/g, average pore size 3.5±1 nm). The mean square surface roughness is 345±30 nm. It was found that KBr also plays the role of a pore former in the system. The dependence of the swelling of the obtained polyelectrolyte films was studied at different pH values corresponding to the pH of the skin (5.5), open (7.2), and infected (8.2) wounds. It was found that the swelling of AC films is pH sensitive. Thus, the film swells the most (~ 300 %) in a weakly alkaline environment and the worst (~ 100 %) in a neutral one. The addition of sodium hyaluronate led to the formation of a denser complex, which levelled the pH sensitivity of swelling. Calcium pantothenate was immobilised in the films by adding a solution of drugs to the formation mixture. Infrared spectroscopy has shown that no covalent bonds are formed between calcium pantothenate and the polyelectrolyte complex. Analysis of release profiles has shown that the kinetics of calcium pantothenate release are best described by the Higuchi model, which is typical for diffusion-controlled drug delivery systems. The release process was pH-sensitive and independent of the polyelectrolyte complex. The obtained polyelectrolyte films demonstrate strong potential as matrices for the development of controlled drug delivery systems.
References
Peng, W., Li, D., Dai, K., Wang, Y., Song, P., Li, H., Tang, P., Zhang, Z., Li, Z., Zhou, Y., Zhou, Y., Zhou, C. (2022). Recent progress of collagen, chitosan, alginate and other hydrogels in skin repair and wound dressing applications. Int. J. Biol. Macromol., 208, 400–408. https://doi.org/10.1016/j.ijbiomac.2022.03.002
Huang, Y. A., Lin, W. J. (2025). Nanoparticles composed of polysaccharide chitosan and oligosaccharide alginate for strengthened transdermal delivery of tacrolimus in atopic dermatitis. Carbohydr. Polym. Technol. Appl., 9(33). https://doi.org/10.1016/j.carpta.2024.100655
Ji, M., Li, J., Li, F., Wang, Y., Man, J., Wang, X., Qiu, Y., Zhang, C., Peng, S., Li, J. (2024). A double cross-linked anisotropic quaternized chitosan/sodium alginate-based wound dressing for rapid drainage of biofluids. Mater. Des., 237, 112567. https://doi.org/10.1016/j.matdes.2023.112567
Masuelli, M. A., Illanes, C. O. (2014). Review of the characterization of sodium alginate by intrinsic viscosity measurements: Comparative analysis between conventional and single point methods. Int. J. BioMater. Sci. Eng., 1(1), 1–11.
Rhein-Knudsen, N., Ale, M. T., Ajalloueian, F., Meyer, A. S. (2017). Characterization of alginates from Ghanaian brown seaweeds: Sargassum spp. and Padina spp. Food Hydrocolloids, 71, 236–244. https://doi.org/10.1016/j.foodhyd.2017.05.016
Spadari, C. de C., Lopes, L. B., Ishida, K. (2017). Potential use of alginate-based carriers as antifungal delivery system. Front. Microbiol., 8, 97. https://doi.org/10.3389/fmicb.2017.00097
Pawar, S. N., Edgar, K. J. (2012). Alginate derivatization: A review of chemistry, properties and applications. Biomaterials, 33(11), 3279–3305. https://doi.org/10.1016/j.biomaterials.2012.01.007
Das, A., Ghosh, S., Pramanik, N. (2024). Chitosan biopolymer and its composites: Processing, properties and applications—A comprehensive review. Hybrid Adv., 6, 100265. https://doi.org/10.1016/j.hybadv.2024.100265
Zhang, X., Liang, Y., Huang, S., Guo, B. (2024). Chitosan-based self-healing hydrogel dressing for wound healing. Adv. Colloid Interface Sci., 332, 103267. https://doi.org/10.1016/j.cis.2024.103267
Muzzarelli, R. A. A. (2009). Chitins and chitosans for the repair of wounded skin, nerve, cartilage and bone. Carbohydr. Polym., 76(2), 167–182. https://doi.org/10.1016/j.carbpol.2008.11.002
Liu, H., Wang, C., Li, C., Qin, Y., Wang, Z., Yang, F., Li, Z., Wang, J. (2018). A functional chitosan-based hydrogel as a wound dressing and drug delivery system in the treatment of wound healing. RSC Adv., 8(14), 7533–7549. https://doi.org/10.1039/c7ra13510f
Atma, Y., Sadeghpour, A., Murray, B. S., Goycoolea, F.M. (2025). Chitosan–alginate polyelectrolyte complexes for encapsulation of low molecular weight fish bioactive peptides. Food Hydrocolloids, 160, 110789. https://doi.org/10.1016/j.foodhyd.2024.110789
Chalitangkoon, J., Wongkittisin, M., Monvisade, P. (2020). Silver loaded hydroxyethylacryl chitosan/sodium alginate hydrogel films for controlled drug release wound dressings. Int. J. Biol. Macromol., 159, 194–203. https://doi.org/10.1016/j.ijbiomac.2020.05.061
Xiao, S., Ahn, D. U. (2022). Enhanced lutein stability under UV-light and high temperature by loading it into alginate–chitosan complex. LWT, 164, 113663. https://doi.org/10.1016/j.lwt.2022.113663
Potaś, J., Szymańska, E., Winnicka, K. (2020). Challenges in developing chitosan–based polyelectrolyte complexes as a platform for mucosal and skin drug delivery. Eur. Polym. J., 140, 110020. https://doi.org/10.1016/j.eurpolymj.2020.110020
Crasta, A., Painginkar, T., Sreedevi, A., Pawar, S. D., Badamane Sathyanarayana, M., Vasantharaju, S. G., Osmani, R. A. M., Ravi, G. (2025). Transdermal drug delivery system: A comprehensive review of innovative strategies, applications, and regulatory perspectives. OpenNano, 24, 100245. https://doi.org/10.1016/j.onano.2025.100245
Di Rago, S., Morandi, F., Pizzetti, F., Rossi, F. (2025). Advances in controlled release systems for sustainable crop production: A review of nano-, micro-, and macro-formulations. Adv. AgroChem., 4 (3), 188-206 https://doi.org/10.1016/j.aac.2025.05.004
Graça, M. F. P., Miguel, S. P., Cabral, C. S. D., Correia, I. J. (2020). Hyaluronic acid–based wound dressings: A review. Carbohydr. Polym., 241, 116364. https://doi.org/10.1016/j.carbpol.2020.116364
Tighsazzadeh, M., Boateng, J. (2024). Matrix hyaluronic acid and bilayer poly-hydroxyethyl methacrylate–hyaluronic acid films as potential ocular drug delivery platforms. Int. J. Biol. Macromol., 260, 129496. https://doi.org/10.1016/j.ijbiomac.2024.129496
Balima, M., Morfin, I., Sudre, G., Montembault, A. (2024). Stretchable hydrogels of chitosan/hyaluronic acid induced by polyelectrolyte complexation around neutral pH. Carbohydr. Polym., 339, 122265. https://doi.org/10.1016/j.carbpol.2024.122265
Galland, P., Iqbal, M. H., Favier, D., Legros, M., Schaaf, P., Boulmedais, F., Vahdati, M. (2024). Tuning the underwater adhesiveness of antibacterial polysaccharides complex coacervates. J. Colloid Interface Sci., 661, 196–206. https://doi.org/10.1016/j.jcis.2024.01.193
Bruschi, M. (2015). Mathematical models of drug release. In Strategies to Modify the Drug Release from Pharmaceutical Systems. https://doi.org/10.1016/B978-0-08-100092-2.00005-9
Manu, G. P., Narh, D., Asimeng, B., Kwakye, N., Abusuapa, B. A., Appuing, D., Nyankson, E., Efavi, J. K. (2025). Characterization of chitosan/zeolite drug delivery composite and curcumin release kinetics in a simulated pH environment. Sci. Afr., 28, e02668. https://doi.org/10.1016/j.sciaf.2025.e02668
Higuchi, T. (1963). Mechanism of sustained-action medication: Theoretical analysis of rate of release of solid drugs dispersed in solid matrices. J. Pharm. Sci., 52(12), 1145–1149. https://doi.org/10.1002/jps.2600521210
Klech, C. M., Simonelli, A. P. (1989). Examination of the moving boundaries associated with non-Fickian water swelling of glassy gelatin beads: Effect of solution pH. J. Membr. Sci., 43(1), 87–101. https://doi.org/10.1016/S0376-7388(00)82355-8
Kamp, J., Emonds, S., Borowec, J., Restrepo Toro, M. A., Wessling, M. (2021). On the organic solvent-free preparation of ultrafiltration and nanofiltration membranes using polyelectrolyte complexation in an all-aqueous phase inversion process. J. Membr. Sci., 618, 118632. https://doi.org/10.1016/j.memsci.2020.118632
Combs, G. F., Jr., McClung, J. P. (2022). Pantothenic acid. In The Vitamins. Elsevier. https://doi.org/10.1016/B978-0-323-90473-5.00009-4
Jia, X., Qian, P., Wu, C., Xie, Y., Yang, W., Song, R., Wu, J., Ye, J. (2022). Effects of dietary pantothenic acid on growth, antioxidant ability and innate immune response in juvenile black carp. Aquac. Rep., 24, 101131. https://doi.org/10.1016/j.aqrep.2022.101131
Sikach, A. V., Konovalova, V. V., Kolesnyk, I. S. (2024). Hydrogel films based on sodium alginate modified with octane-1-amine: Enhanced pore formation and potential applications in drug delivery systems. Himia Fiz. Tehnol. Poverhni, 15(1), 43–56. https://doi.org/10.15407/hftp15.01.043
Gregg, S. J. (1982). Adsorption, surface area, and porosity. London, UK: Academic Press.
Sikach, A., Bubela, H., Konovalova, V., Kolesnyk, I. (2024). Porous sodium alginate hydrogel films for immediate release drug delivery systems. Chem. Chem. Technol., 18(4), 524–534. https://doi.org/10.23939/chcht18.04.524
Konovalova, V., Kolesnyk, I., Savchenko, M., Marynin, A., Bubela, H., Kujawa, J., Knozowska, K., Kujawski W. (2023). Preparation of chitosan water-in-oil emulsions by stirred cell membrane emulsification. Colloids Surf. A Physicochem. Eng. Asp., 661, 130929. https://doi.org/10.1016/j.colsurfa.2023.130929
Downloads
Published
Issue
Section
License
Copyright (c) 2025 Oles Honchar Dnipro National University

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

