ALKYLATION OF SUBSTITUTED SULFONAMIDES WITH BENZOTRICHLORIDE AND IPSO-SUBSTITUTION OF THE p-TOLUENESULFONAMIDE GROUP WITH NUCLEOPHILIC REAGENTS
DOI:
https://doi.org/10.15421/jchemtech.v33i3.332919Keywords:
sulfonamides, N-alkylation, benzotrichloride, ipso-substitution, aluminum chloride (AlCl₃), electrophilic catalysis, amines, pharmaceutical chemistryAbstract
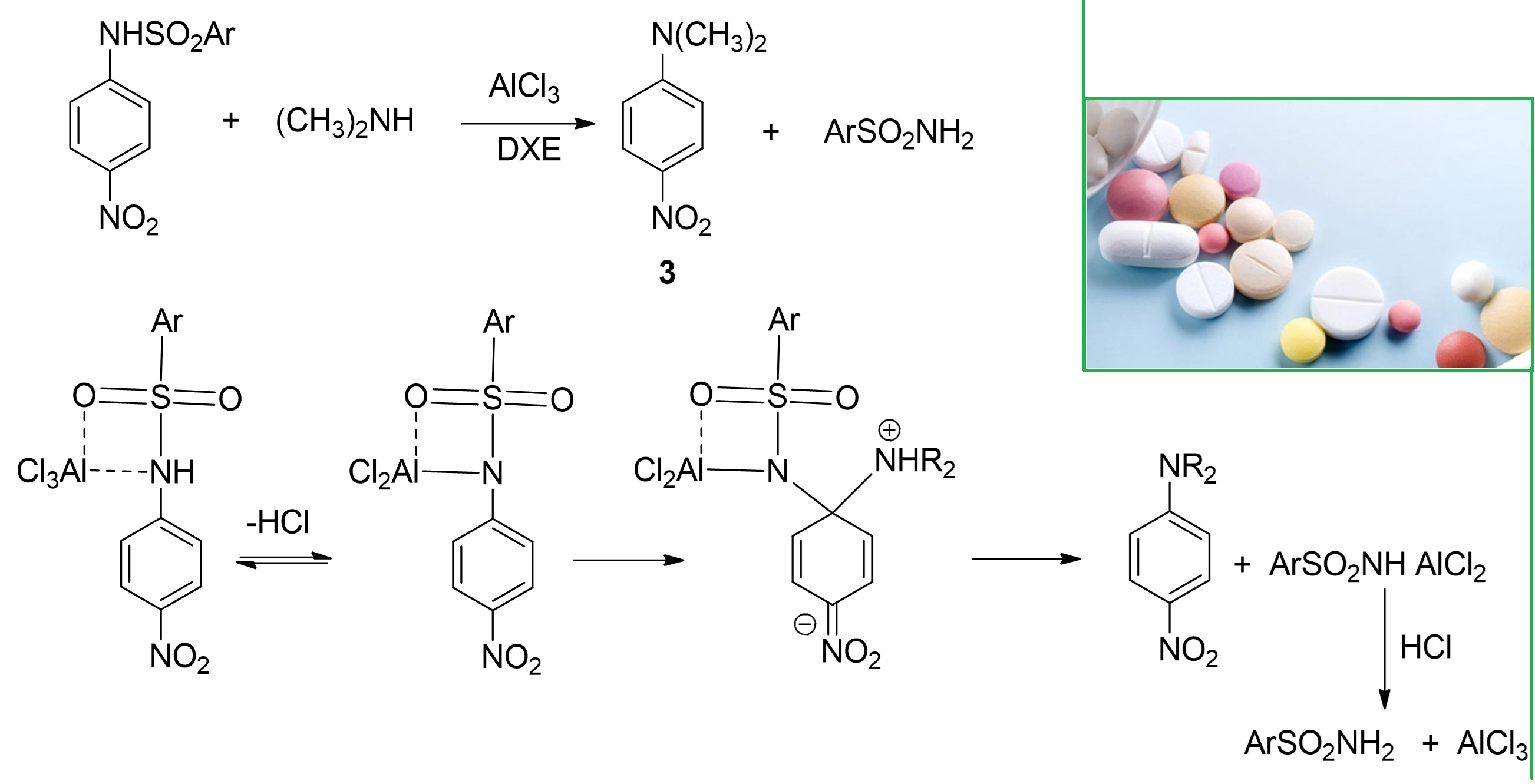
Substituted alkyl and aryl sulfonamides have attracted increasing research interest due to their distinctive chemical reactivity. In particular, the arylsulfonyl moiety functions as an excellent leaving group (hypernucleofuge), making these compounds highly electrophilic and suitable for various aromatic substitution and transfer reactions. In this study, we explored the alkylation of substituted sulfonamides using polyhaloalkanes such as benzotrichloride. This reaction yielded imidoyl chlorides, which are important intermediates in the synthesis of natural products, pharmaceuticals, biologically active compounds, and advanced materials. Additionally, under specific conditions - including the presence of AlCl₃, 1,2-dichloroethane, and strong amines - we observed the replacement of the sulfonamide group by an amine via ipso-substitution. We investigated the synthesized compounds using infrared (IR) and nuclear magnetic resonance (NMR 1Н) spectroscopy, thin-layer chromatography (TLC) and elemental analysis. These techniques confirmed the structures and purity of the reaction products. Alkylation proceeded efficiently only in the presence of benzotrichloride and AlCl₃; other polyhalogenated reagents and Lewis’s acids exhibited no reactivity under comparable conditions. Furthermore, ipso-substitution of the sulfonamide group by strong nucleophilic amines (pKa > 10), such as dimethylamine and piperidine, occurred even in the absence of benzotrichloride. A nitro group in the para position of the arylamine moiety was essential for selective ipso-substitution, due to its activating effect on the aromatic system. Both electronic and steric factors were found to significantly influence the reaction pathway. Substituting the nitro group with other electron-withdrawing groups or placing it in non-para-positions significantly reduced the rate of the substitution process.
References
Ovung, A., Bhattacharyya, J. (2021). Sulfonamide drugs: Structure, antibacterial property, toxicity, and biophysical interactions. Biophys. Rev., 13(2), 259–272. https://doi.org/10.1007/s12551-021-00795-9
Brzeski, J., Ciesielska, A., Makowski, M. (2023). Theoretical study on the alkylimino-substituted sulfonamides with potential biological activity. J. Phys. Chem. B, 127(30), 6620–6627. https://doi.org/10.1021/acs.jpcb.3c01965
El-Gaby, M., Ammar, Y. A., El-Qaliei, M. I. H., Hussein, M. F., Faraghally, F. A. (2020). Sulfonamides: Synthesis and the recent applications in medicinal chemistry. Egyp. J. Chem., 63(12), 5289–5327. https://doi.org/10.21608/ejchem.2020.33860.2707
Babalola, I. T., Suleiman, G. (2024). Design, synthesis, and molecular docking studies of N-substituted sulfonamides as potential anticancer therapeutics. J. Taibah Univ. Med. Sci., 19(1), 175–183. https://doi.org/10.1016/j.jtumed.2023.10.006
Mussi, S., Rezzola, S., Chiodelli, P., Nocentini, A., Supuran, C. T., Ronca, R. (2022). Antiproliferative effects of sulphonamide carbonic anhydrase inhibitors C18, SLC-0111 and acetazolamide on bladder, glioblastoma and pancreatic cancer cell lines. J. Enzym. Med. Chem., 37(1), 280–286. https://doi.org/10.1080/14756366.2021.2004592
Qu, B., Xu, Y, Lu, Y., Zhuang, W., Jin, X., Shi, Q., Shike, Y., Guo, Y., Shen, Z., Che, J., Wu, Y., Tong, L., Dong, X., Yang, H. (2022). Design, synthesis and biological evaluation of sulfonamides inhibitors of XPO1 displaying activity against multiple myeloma cells. Eur. J. Med. Chem., 235, 114257. https://doi.org/10.1016/j.ejmech.2022.114257
Butt, A. F., Ahmed, M. N., Bhatti, M. H., Choudhary, M. A., Ayub, K., Tahir, M. N., Mahmood, T. (2019). Synthesis, structural properties, DFT studies, antimicrobial activities and DNA binding interactions of two newly synthesized organotin (IV) carboxylates. J. Mol. Struct., 1191, 291–300. https://doi.org/10.1016/j.molstruc.2019.04.066
Martinez-Asencio, A., Ramon, D. J., Yus, M. (2011). N-Alkylation of poor nucleophilic amines and derivatives with alcohols by a hydrogen autotransfer process catalyzed by copper (II) acetate: scope and mechanistic considerations. Tetrahedron, 67(17), 3140–3149. https://doi.org/10.1016/j.tet.2011.02.075
Yun, X. J., Ling, C., Deng, W., Liu, Z. J., Yao, Z. J. (2020). Half-sandwich Ru (II) complexes with N, O-chelate ligands: diverse catalytic activity for amine synthesis in water. Organometallics, 39(21), 3830–3838. https://doi.org/10.1021/acs.organomet.0c00554
Yiğit, B., Karaca, E. Ö., Yiğit, M., Gürbüz, N., Arslan, H., Özdemir, İ. (2020). Active ruthenium (II)-NHC complexes for alkylation of amines with alcohols using solvent-free conditions. Polyhedron, 175, 114234. https://doi.org/10.1016/j.poly.2019.114234
Balamurugan, G., Ramesh, R., Malecki, J. G. (2020). Nickel (II)–NΛNΛO pincer type complex-catalyzed N-alkylation of amines with alcohols via the hydrogen autotransfer reaction. J. Org. Chem., 85(11), 7125–7135. https://doi.org/10.1021/acs.joc.0c00530
Niu, F., Wang, Q., Yan, Z., Kusema, B. T., Khodakov, A. Y., Ordomsky, V. V. (2020). Highly efficient and selective N-Alkylation of amines with alcohols catalyzed by in situ rehydrated titanium hydroxide. ACS Catal., 10(5), 3404–3414. https://doi.org/10.1021/acscatal.9b05525
Wu, W., Rao, W., Er, Y. Q., Loh, J. K., Poh, C. Y., Chan, P.W.H. (2008). Iodine-catalyzed allylic alkylation of sulfonamides and carbamates with allylic alcohols at room temperature. Tetrahedron Lett., 49(16), 2620–2624. https://doi.org/10.1016/j.tetlet.2008.02.079
Bhatt, S., Rana, M., Sharma, A. K., Joshi, H. (2023). Ruthenium Complexes of Bidentate N, N‐Ligand as Catalyst for Selective N‐Alkylation of Amines with Alcohols. Asian J. Org. Chem., 12(6), e202300158. https://doi.org/10.1002/ajoc.202300158
Echeverry-Gonzalez, C. A., Kouznetsov, V. V. (2021). Pursuit for simple and efficient ligands promoting copper-catalyzed Ullmann type reactions for N-aryl heterocycles and aromatic amines. In Copper in N-Heterocyclic Chemistry. Elsevier, India, (Chapter 11), 399–430. https://doi.org/10.1016/B978-0-12-821263-9.00011-4
Murali, A., Puppala, M., Varghese, B., Baskaran, S. (2011). A lewis acid mediated schmidt reaction of benzylic azide: synthesis of sterically crowded aromatic tertiary amines. Eur. J. Org. Chem., 2011(27), 5297–5302 https://doi.org/10.1002/ejoc.201100674
Shi, W., Bai, C. M., Zhu, K., Cui, D. M., Zhang, C. (2014). Brønsted acid-assisted N-alkylation of sulfonamides using ethers as the alkylation reagents. Tetrahedron, 70(2), 434-438. https://doi.org/10.1016/j.tet.2013.11.036
Li, Y. Q., Chen, Y. B., Huang, Z. Z. (2014). Direct N-alkylation of amines with alcohols using AlCl3 as a Lewis acid. Chin. Chem. Lett., 25(12), 1540–1544. http://doi.org/10.1016/j.cclet.2014.07.006
Benali, N., Bougheloum, C., Alioua, S., Belghiche, R., Messalhi, A. (2018). Efficient N-acylation of sulfonamides using cesium salt of Wells–Dawson heteropolyacid as catalyst: Synthesis of new N-acyl sulfonamides and cyclic imides. Synthetic Communications, 48(24), 3099–3112. https://doi.org/10.1080/00397911.2018.1535077
Дмітрікова, Л. В., Коптєва, С. Д., Марков, В. І. (2016). N-алкілування сульфонамідів дигалоїдалкілами в умовах електрофільного каталізу та перетворення алкільованих сполук. Bulletin of Dnipropetrovsk University. Series Chemistry, 24(2), 73–80. https://doi.org/10.15421/081610
da Mata, M. L. E., Motherwell, W. B., Ujjainwalla, F. (1997). Steric and electronic effects in the synthesis of biaryls and their heterocyclic congeners using intramolecular free radical [1, 5] ipso substitution reactions. Tetrahedron lett., 38(1), 137–140. https://doi.org/10.1016/S0040-4039(96)02236-8
Müller, P., Phuong, N. T. M. (1979). ipso‐Substitution with sodium‐N‐alkyl‐(p‐nitrobenzene) sulfonamide. A novel anionic rearrangement. Helv. Chim. Acta, 62(2), 494–496. https://doi.org/10.1002/hlca.19790620215
Ryokawa, A., Togo, H. (2001). Synthetic use of 1, 1, 2, 2-tetraphenyldisilane for the preparation of biaryls through the intramolecular free radical ipso-substitution of N-(2-bromoaryl)arenesulfonamides. Tetrahedron, 57(28), 5915–5921. https://doi.org/10.1016/S0040-4020(01)00560-9
Chen, H., Wang, L., Han, J. (2020). Aryl radical-induced desulfonylative ipso-substitution of diaryliodonium salts: an efficient route to sterically hindered biarylamines. Chem. Commun., 56(42), 5697–5700. https://doi.org/10.1039/D0CC01766C
Mondal, S., Roy, S., Sikdar, A., Bodar, A., Paul, A., Mandal, B. (2024). Intermolecular Ipso Aromatic Nucleophilic Substitution of Electron‐Deficient Aryl Sulfonyl Chlorides. Chemistry Select, 9(42), e202403634. https://doi.org/10.1002/slct.202403634
СhemicalBook. CAS DataBase List https://www.chemicalbook.com
Downloads
Published
Issue
Section
License
Copyright (c) 2025 Oles Honchar Dnipro National University

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

