DIMERIZATION OF MALEINATE CHLOROCOMPLEXES OF Cu+. QUANTUM-CHEMICAL MODELING
DOI:
https://doi.org/10.15421/jchemtech.v33i3.333798Keywords:
maleate aquachlorocomplexes, Cu , dimerization, quantum chemical modelingAbstract
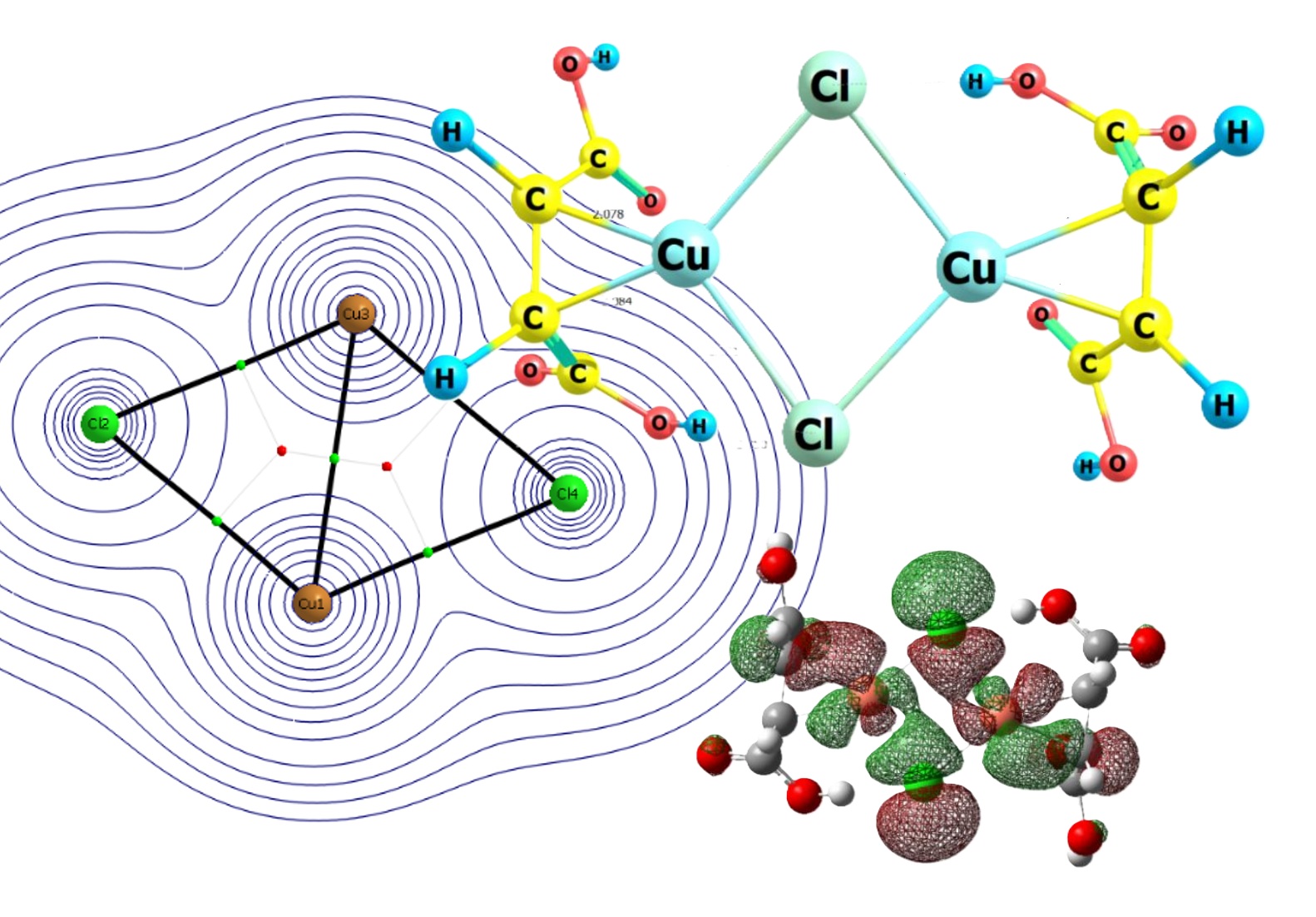
The article presents the results of a theoretical study of the dimerization processes of Cu+ maleate complexes in an aqueous medium in the presence of chlorine anions. Quantum-chemical modeling by the DFT method (Gaussian 09, AIM2000) showed that the synthesis of Cu+ maleate π-complexes using CuCl salt as a precursor can be accompanied by dimerization of the product. Moreover, in addition to [L-Cu2Cl2-L] dimers, [H2O-Cu2Cl2-H2O] dimers can also be formed in solution. Consideration of the probable reactions of the interaction of maleic acid with dimeric aquachloro complexes allowed us to establish that the dimeric core cannot simultaneously contain both water and maleic acid in its internal coordination sphere. Comparison of the energy parameters of various reactions involving the [Cu2Cl2] cluster, water molecules, and all forms of maleic acid indicates that the most energetically favorable structures are dimers of anionic complexes. Therefore, in a weakly acidic environment, one should expect the appearance of [HM-Cu2Cl2-HM]2– ions, and in neutral and alkaline environments, [M-Cu2Cl2-M]4– ions. It has been established that the addition of all forms of maleic acid to [Cu2Cl2] leads to the rupture of the Cu-Cu bond and, as a consequence, to the weakening of this core in [L-Cu2Cl2-L] dimers.
References
Wang, X. S., Zhao, H., Li, Y. H., Xiong, R. G., You, X. Z. (2005). Olefin-copper (I) complexes and their properties. Topics in Catalysis, 35, 43–61. https://doi.org/10.1007/s11244-005-3812-6
Ashraf, J., Riaz, M. A. (2022). Biological potential of copper complexes: a review. Turkish Journal of Chemistry, 46(3), 595–623. https://doi.org/10.55730/1300-0527.3356
Harmalkar, S.S., Butcher, R. J., Gobre, V. V., Gaonkar, S. K., D’Souza, L. R., Sankaralingam, M., Furtado, І., Dhuri, S. N. (2019). Synthesis, characterization and antimicrobial properties of mononuclear copper (II) compounds of N,N′-di(quinolin-8-yl)cyclohexane-1,2-diamine. Inorganica Chimica Acta, 498, 119020. https://doi.org/10.1016/j.ica.2019.119020
Santiago, P. H., Santiago, M. B., Martins, C. H., Gatto, C. C. (2020). Copper (II) and zinc (II) complexes with Hydrazone: Synthesis, crystal structure, Hirshfeld surface and antibacterial activity. Inorganica Chimica Acta, 508, 119632. https://doi.org/10.1016/j.ica.2020.119632
Sharma, R. P., Kumar, S., Venugopalan, P., Gondil, V. S., Chhibber, S., Jezierska, J., Ferretti, V. (2016). Effect of heterocyclic nitrogen donor ligands on coordination behavior of weakly coordinating arylsulfonate: Synthesis, characterization and antimicrobial activities of [Cu(β-pic)4(2-Cl-5-nitrobenzenesulfonate)2]-(methanol) and [Cu(γ-pic)4(2-Cl-5 nitrobenzene-sulfonate)2]. Inorganica Chimica Acta, 449, 52–60. https://doi.org/10.1016/j.ica.2016.04.049
Urquiza, N. M., Islas, M. S., Dittler, M. L., Moyano, M. A., Manca, S. G., Lezama, L., Rojo, T., Medina, J. J. M., Diez, M., Tévez, L. L., Williams, P. A. M., Ferrer, E. G. (2013). Inhibition behavior on alkaline phosphatase activity, antibacterial and antioxidant activities of ternary methimazole–phenanthroline–copper(II) complex. Inorganica Chimica Acta, 405, 243–251. https://doi.org/10.1016/j.ica.2013.05.022
Soroceanu, A., Vacareanu, L., Vornicu, N., Cazacu, M., Rudic, V., Croitori, T. (2016). Assessment of some application potentials for copper complexes of the ligands containing siloxane moiety: Antimicrobial, antifungal, antioxidant and redox activity. Inorganica Chimica Acta, 442, 119–123. https://doi.org/10.1016/j.ica.2015.12.006
Kumar, S., Sharma, R. P., Venugopalan, P., Gondil, V. S., Chhibber, S., Aree, T., Witwicki, M., Ferretti, V. (2018). Hybrid inorganic-organic complexes: Synthesis, spectroscopic characterization, single crystal X-ray structure determination and antimicrobial activities of three copper(II)-diethylenetriamine-p-nitrobenzoate complexes. Inorganica Chimica Acta, 469, 288–297. https://doi.org/10.1016/j.ica.2017.09.032
Lima, F. C., Silva, T. S., Martins, C. H., Gatto, C. C. (2018). Synthesis, crystal structures and antimicrobial activity of dimeric copper (II) complexes with 2-hydroxyphenyl-ethylidene-dithiocarbazates. Inorga-nica Chimica Acta, 483, 464–472. https://doi.org/10.1016/j.ica.2018.08.032
Beheshti, A., Nozarian, K., Babadi, S. S., Noorizadeh, S., Motamedi, H., Mayer, P., Bruno, G., Rudbari, H. A. (2017). Structural variability in Cu(I) and Ag(I) coordination polymers with a flexible dithione ligand: Synthesis, crystal structure, microbiological and theoretical studies. Journal of Solid State Chemistry, 249, 70–79. https://doi.org/10.1016/j.jssc.2016.12.013
González-Ballesteros, N., Pérez-Álvarez, D., Rodríguez-Argüelles, M. C., Henriques, M. S., Paixão, J. A., Prado-López, S. (2016). Synthesis, spectral characterization and X-ray crystallographic study of new copper(I) complexes. Antitumor activity in colon cancer. Polyhedron, 119, 112–119. https://doi.org/10.1016/j.poly.2016.08.023
Zhang, Z., Wang, H., Wang, Q., Yan, M., Wang, H., Bi, C., Sun, S., Fan, Y. (2016). Anticancer activity and computational modeling of ternary copper (II) complexes with 3-indolecarboxylic acid and 1, 10-phenanthroline. International Journal of Oncology, 49(2), 691–699. https://doi.org/10.3892/ijo.2016.3542
Marzano, C., Pellei, M., Tisato, F., Santini, C. (2009). Copper complexes as anticancer agents. Anti-Cancer Agents in Medicinal Chemistry-Anti-Cancer Agents, 9(2), 185–211. https://doi.org/10.2174/187152009787313837
Vargalyuk, V.F., Polonskyy, V. A., Sklyar, T. V., Stets, N. V., Laguta O. V. (2023). Physico-chemical and bactericidal properties of copper-containing composites based on maleinate complexes Cu+. Journal of Chemistry & Technologies, 31(2), 208–215. https://doi.org/10.15421/jchemtech.v31i2.275070
Pavliuk, O., Goreshnik, E. (2019). [Synthesis and crystal structure of heterohalogenated π-complexes of Cu(I) with 1, 3-dialylbenzimidazolone]. Bulletin of Lviv University. Chemical series, 60(1), 170–178. (In Ukrainian).
Yang, L., Powell, D. R., Houser, R. P. (2007). Structural variation in copper(I) complexes with pyridylmethylamide ligands: structural analysis with a new four-coordinate geometry index, τ 4. Dalton Transactions, (9), 955–964. https://doi.org/10.1039/B617136B
Slyvka, Yu. (2017). [Peculiarities of construction of crystalline π-complexes of copper (I) benzene sulfonate with 2-amino-5-allylthio-1,3,4-thiadiazole]. Bulletin of Lviv University. Chemical series, 58(1), 172–180. (In Ukrainian).
Ardan, B., Kinzhybalo, V., Slyvka, Y., Shyyka, O., Lukyanov, M., Lis, T., Myskiv, M. (2017). Ligand-forced dimerization of copper(I)–olefin complexes bearing a 1,3,4-thiadiazole core. Crystal Structure Communications, 73(1), 36–46. https://doi.org/10.1107/S2053229616018751
Slyvka, Y. I., Fedorchuk, A. A., Pokhodylo, N. T., Lis, T., Kityk, I. V., Mys’ kiv, M. G. (2018). A novel copper(I) sulfamate π-complex based on the 5-(allylthio)-1-(3, 5-dimethylphenyl)-1H-tetrazole ligand: Alternating-current electrochemical crystallization, DFT calculations, structural and NLO properties studies. Polyhedron, 147, 86–93. https://doi.org/10.1016/j.poly.2018.03.015
Slyvka, Yu. (2019). [π- complexes of copper(i) chloride and copper(i) perchlorate with 2-allylthio-5-methyl-1,3,4-thiadiazole: synthesis and crystal structure]. Bulletin of Lviv University. Chemical series, 60(1), 155–162. (In Ukrainian).
Lukyanov, M., Slyvka, Y., Ardan, B., Miskiv, M. (2018). [Synthesis and crystal structure of a π-complex of copper(I) sulfamate with 32-(N-allyl)-amino-5-methyl-1,3,4-thiadiazole composition [Cu2(C6H10N3S2)2(NH2SO3)2].]. Bulletin of Lviv University. Chemical series, 59(1), 157–163. (In Ukrainian).
Vargalyuk, V. F., Osokin, Y. S., Polonskyy, V. A., Glushkov, V. N. (2019). Features of (dπ-pπ)-binding of Cu(I) ions with acrylic, maleic and fumaric acids in aqueous solution. Journal of Chemistry and Technologies, 27(2), 148–157. https://doi.org/10.15421/081916
Vargalyuk, V. F., Osokin, Y. S., Polonskyy, V. A. (2020). Formation of the π-complexes of copper atoms with acrylic, maleic and fumaric acids in aqueous medium. Journal of Chemistry and Technologies, 28(2), 153–116. https://doi.org/10.15421/082016
Kurasova, Y. D., Vargalyuk, V. F., Polonskyy, V. A. (2022). Quantum chemical modeling of aquachlorocomplexes of Cu+ with acrylic, maleic and fumaric acids. Journal of Chemistry and Technologies, 30(4), 530–536. https://doi.org/10.15421/jchemtech.v30i4.263280
Goreshnik, E. A., Veryasov, G., Morozov, D., Slyvka, Y., Ardan, B., Mys’ kiv, M. G. (2016). Solvated copper(I) hexafluorosilicate π-complexes based on [Cu2(amtd)2]2+(amtd = 2-allylamino-5-methyl-1,3,4-thiadiazole) dimer. Journal of Organometallic Chemistry, 810, 1–11. https://doi.org/10.1016/j.jorganchem.2016.03.001
Chemcraft. (n.d.). Chemcraft – graphical software for visualization of quantum chemistry computations (Version 1.8, build 682). https://www.chemcraftprog.com
Frisch, M. J. E. A. (2009). Gaussian 09, Revision D. 01, Gaussian." Inc, Wallingford CT 201.
Cortés-Guzmán, F., Bader, R. F. (2005). Complementarity of QTAIM and MO theory in the study of bonding in donor–acceptor complexes. Coordination Chemistry Reviews, 249(5-6), 633–662. https://doi.org/10.1016/j.ccr.2004.08.022
Biegler-Konig, F., Schonbohm, J., Bayles, D. (2001). Software news and updates-AIM2000-A program to analyze and visualize atoms in molecules. Journal of Computational Chemistry, 22(5), 545–559.
Tomasi, J., Mennucci, B., Cammi, R. (2005). Quantum mechanical continuum solvation models. Chemical reviews, 105(8), 2999–3094. https://doi.org/10.1021/cr9904009
Espinosa, E., Molins, E., Lecomte, C. (1998). Hydrogen bond strengths revealed by topological analyses of experimentally observed electron densities. Chemical physics letters, 285(3-4), 170–173. https://doi.org/10.1016/S0009-2614(98)00036-0
Vargaljuk, V., Okovytyy, S., Polonskyy, V., Kramska, O., Shchukin, A., Leszczynski, J. (2017). Copper crystallization from aqueous solution: initiation and evolution of the polynuclear clusters. Journal of Cluster Science, 28(5), 2517–2528. https://doi.org/10.1007/s10876-017-1239-4
Pratiwi, R., Ibrahim, S., Tjahjono, D. H. (2020). Reactivity and stability of metalloporphyrin complex formation: DFT and experimental study. Molecules, 25(18), 4221. https://doi.org/10.3390/molecules25184221
Downloads
Published
Issue
Section
License
Copyright (c) 2025 Oles Honchar Dnipro National University

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

