SYNTHESIS, X-RAY CRYSTAL STRUCTURE, SPECTROSCOPIC CHARACTERIZATION AND MALDI MASS SPECTRA, HIRSHFELD SURFACE ANALYSIS OF OCTANUCLEAR AZAMETALLACROWN COPPER(II) COMPLEX WITH 3,5-DIMETHYL-1H-PYRAZOLE, OBTAINED BY OXIDATIVE DISSOLUTION METHOD
DOI:
https://doi.org/10.15421/jchemtech.v33i4.334651Keywords:
pyrazole ligands, copper complexes, oxidative dissolution, X-ray crystallography, metallacrown, Hirshfeld surface analysisAbstract
A novel copper(II) polynuclear complex with deprotonated 3,5-dimethyl-1H-pyrazole of composition
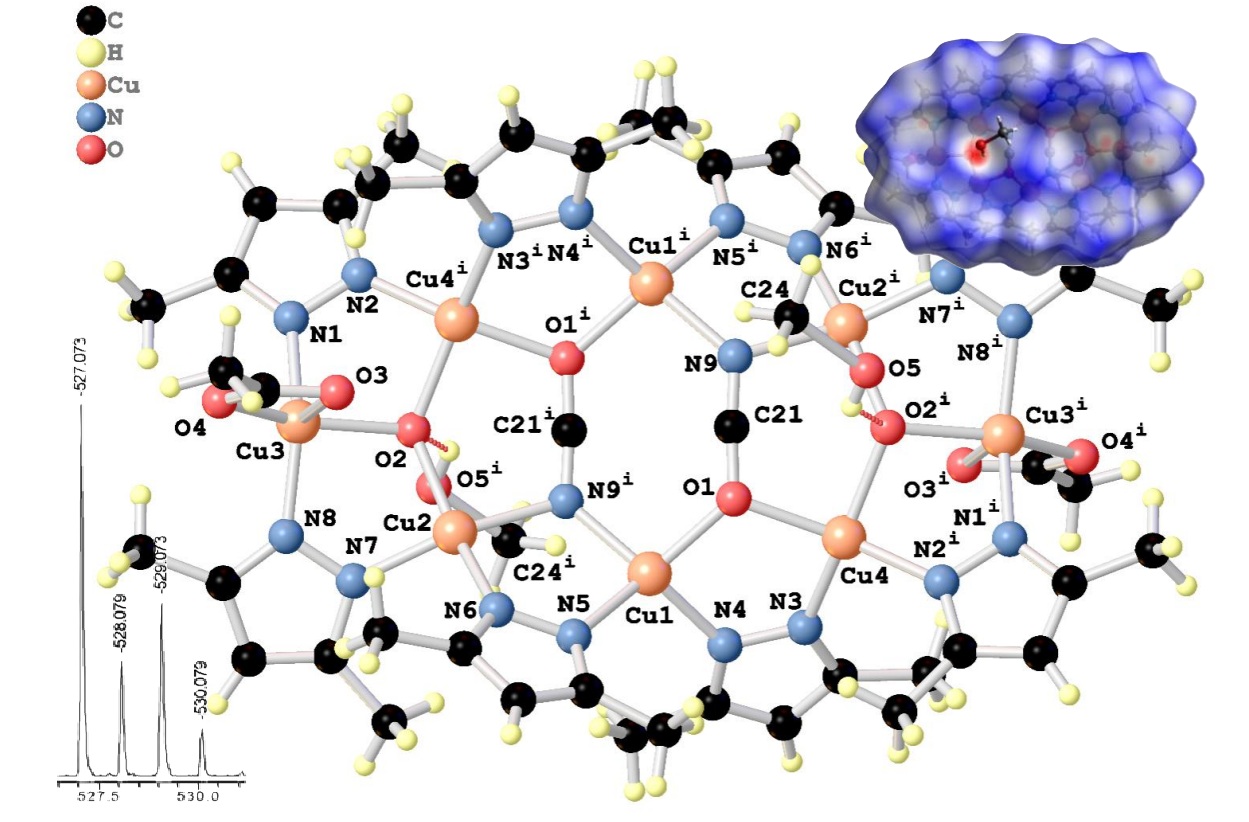
Cu8(µ3-O)2(DMPZ-Н)8(NCO)2(OAc)2·2CH3OH has been synthesized and isolated in the crystalline state. A variety of techniques were employed to identify and characterize the structure of the complex, including IR spectroscopy, microanalysis, MALDI analysis, and single-crystal X-ray diffraction. It was observed that Cu8(µ3-O)2(DMPZ-Н)8(NCO)2(OAc)2·2CH3OH had been formed as a result of a multistage process of oxidative dissolution of metallic copper with the participation of air oxygen and ammonium ions. This process results in the accumulation of a sufficient concentration of Cu²⁺ ions in the solution, which contributes to both the formation of the final product and the dissolution of metallic copper through the intermediate generation of Cu⁺ species. These intermediates are subsequently oxidized by atmospheric oxygen to yield divalent copper compounds. The resulting octanuclear complex Cu8(µ3-O)2(DMPZ-Н)8(NCO)2(OAc)2·2CH3OH is a new inverted 24-azametallocrown-8, due to the implementation of the cyclic interaction (-Cu-N-N-)8 formed by eight copper ions and eight μ2-bridge coordinated 3,5-dimethyl-1H-pyrazole anions. A crystallographic 2-fold axis bisects the obtained octanuclear complex on the same two trinuclear fragments Cu2-Cu3-Cu4i and Cu2i-Cu3i-Cu4, which are sequentially linked by deprotonated bidentate-bridging coordinated molecules of 3,5-dimethyl-1H-pyrazoles and bridging μ3-O2-, which are the centers of coordination for three copper cations. Tetradentate-bridged cyanate groups additionally assemble the thrinuclear fragments into an octanuclear complex. Hirshfeld surface analysis, complemented by two-dimensional fingerprint plots, was carried out to investigate the intermolecular interactions in the crystal structure. The results revealed dominant H···H contacts (71.7 %) along with notable contributions from H···C/C···H, H···O/O···H and H···N/N···H interactions, confirming that hydrogen bonding plays a significant role in stabilizing the crystal packing.
References
Yusof, Z., Ramasamy, S., Mahmood, N. Z., Yaacob, J. S. (2018). Vermicompost supplementation improves the stability of bioactive anthocyanin and phenolic compounds in Clinacanthus nutans Lindau. Molecules, 23(6), 1345. https://doi.org/10.3390/molecules23061345
Ameziane El Hassani, I., Rouzi, K., Assila, H., Karrouchi, K., Ansar, M. H. (2023). Recent advances in the synthesis of pyrazole derivatives: a review. Reactions, 4(3), 478–504. https://doi.org/10.3390/reactions4030029
Ebenezer, O., Shapi, M., Tuszynski, J. A. (2022). A review of the recent development in the synthesis and biological evaluations of pyrazole derivatives. Biomedicines, 10(5), 1124. https://doi.org/10.3390/biomedicines10051124
Menezes, R. A., Bhat, K. S. (2025). Synthetic aspects, structural insights and pharmacological potential of pyrazole derivatives: an overview. Discover Applied Sciences, 7(2), 137. https://doi.org/10.1007/s42452-025-06528-x
Ansari, A., Ali, A., Asif, M. (2017). Biologically active pyrazole derivatives. New Journal of Chemistry, 41(1), 16-41. https://doi.org/10.1039/C6NJ03181A
Abdellatif, K. R., Fadaly, W. A., Elshaier, Y. A., Ali, W. A., Kamel, G. M. (2018). Non-acidic 1, 3, 4-trisubstituted-pyrazole derivatives as lonazolac analogs with promising COX-2 selectivity, anti-inflammatory activity and gastric safety profile. Bioorganic Chemistry, 77, 568–578. https://doi.org/10.1016/j.bioorg.2018.02.018
Onoa, G. B., Moreno, V. (2002). Study of the modifications caused by cisplatin, transplatin, and Pd (II) and Pt (II) mepirizole derivatives on pBR322 DNA by atomic force microscopy. International Journal of Pharmaceutics, 245(1-2), 55–65. https://doi.org/10.1016/S0378-5173(02)00332-0
Porcu, A., Melis, M., Turecek, R., Ullrich, C., Mocci, I., Bettler, B., Castelli, M. P. (2018). Rimonabant, a potent CB1 cannabinoid receptor antagonist, is a Gαi/o protein inhibitor. Neuropharmacology, 133, 107–120. https://doi.org/10.1016/j.neuropharm.2018.01.024
Shi, C., Ma, C., Ma, H., Zhou, X., Cao, J., Fan, Y., Huang, G. (2016). Copper-catalyzed synthesis of 1, 3, 4-trisubstituted and 1, 3, 4, 5-tetrasubstituted pyrazoles via [3+ 2] cycloadditions of hydrazones and nitroolefins. Tetrahedron, 72(27-28), 4055–4058. https://doi.org/10.1016/j.tet.2016.05.034
Hughes, A., Hendrickson, R. G., Chen, B. C. C., Valento, M. (2018). Severe loperamide toxicity associated with the use of cimetidine to potentiate the “high”. The American journal of emergency medicine, 36(8), 1527-e3. https://doi.org/10.1016/j.ajem.2018.05.025
Huang, Q., Tran, G., Pardo, D. G., Tsuchiya, T., Hillebrand, S., Vors, J. P., Cossy, J. (2015). Palladium-catalyzed phosphonylation of pyrazoles substituted by electron-withdrawing groups. Tetrahedron, 71(39), 7250–7259. https://doi.org/10.1016/j.tet.2015.03.099
Mullins, K. B., Thomason, J. M., Lunsford, K. V., Pinchuk, L. M., Langston, V. C., Wills, R. W., Mackin, A. J. (2012). Effects of carprofen, meloxicam and deracoxib on platelet function in dogs. Veterinary Anaesthesia and Analgesia, 39(2), 206–217. https://doi.org/10.1111/j.1467-2995.2011.00684.x
El-Din, M. M. G., El-Gamal, M. I., Abdel-Maksoud, M. S., Yoo, K. H., Baek, D., Choi, J., Oh, C. H. (2016). Design, synthesis, and in vitro antiproliferative and kinase inhibitory effects of pyrimidinylpyrazole derivatives terminating with arylsulfonamido or cyclic sulfamide substituents. Journal of enzyme inhibition and medicinal chemistry, 31(sup2), 111–122. https://doi.org/10.1080/14756366.2016.1190715
[Xing, Q., Jiang, D., Zhang, J., Guan, L., Li, T., Zhao, Y., Zhu, Z. (2022). Combining visible-light induction and copper catalysis for chemo-selective nitrene transfer for late-stage amination of natural products. Communications Chemistry, 5(1), 79. https://doi.org/10.1038/s42004-022-00692-6
Davydenko, Y. M., Demeshko, S., Pavlenko, V. A., Dechert, S., Meyer, F., Fritsky, I. O. (2013). Synthesis, crystal structure, spectroscopic and magnetically study of two copper (II) complexes with pyrazole ligand. Zeitschrift für anorganische und allgemeine Chemie, 639(8-9), 1472-1476. https://doi.org/10.1002/zaac.201300078
Cañón-Mancisidor, W., Hermosilla-Ibáñez, P., Spodine, E., Paredes-García, V., Gómez-García, C. J., Venegas-Yazigi, D. (2023). Spin Frustrated Pyrazolato Triangular CuII Complex: Structure and Magnetic Properties, an Overview. Magnetochemistry, 9(6), 155. https://doi.org/10.3390/magnetochemistry9060155
Davydenko, Y., Pavlenko, V., Fritsky, I., Vynohradov, O. (2022). Synthesis, x-ray crystal structure, spectroscopic characterization and hirshfeld surface analysis of dichloro-bis (3,5-dimethyl-4-amino-1h-pyrazole) cobalt (II). Ukrainian Chemistry Journal, 88(6), 127–136. https://doi.org/10.33609/2708-129X.88.06.2022.127-136
Mandal, N. K., Nandi, S., Souilamas, S. B., Garcia, C. J. G., Acharya, K., Naskar, J. P. (2024). Design, synthesis and structure of a trinuclear copper (II) complex having Cu3OH core with regard to aspects of antiproliferative activity and magnetic properties. New Journal of Chemistry. https://doi.org/10.1039/D3NJ04859D
Watanabe, Y., Washer, B. M., Zeller, M., Savikhin, S., Slipchenko, L. V., Wei, A. (2022). Copper (I)–Pyrazolate Complexes as Solid-State Phosphors: Deep-Blue Emission through a Remote Steric Effect. Journal of the American Chemical Society, 144(23), 10186–10192. https://doi.org/10.1021/jacs.1c13462
Shi, K., Mathivathanan, L., Boudalis, A. K., Turek, P., Chakraborty, I., Raptis, R. G. (2019). Nitrite Reduction by Trinuclear Copper Pyrazolate Complexes: An Example of a Catalytic, Synthetic Polynuclear NO Releasing System. Inorganic Chemistry, 58(11), 7537–7544. https://doi.org/10.1021/acs.inorgchem.9b00748
Bagnarelli, L., Dolmella, A., Santini, C., Vallesi, R., Giacomantonio, R., Gabrielli, S., Pellei, M. (2021). A new dimeric Copper (II) complex of hexyl bis (pyrazolyl) acetate ligand as an efficient catalyst for allylic oxidations. Molecules, 26(20), 6271. https://doi.org/10.3390/molecules26206271
Titi, A., Zaidi, K., Alzahrani, A. Y., El Kodadi, M., Yousfi, E. B., Moliterni, A., Abboud, M. (2023). New in situ catalysts based on nitro functional pyrazole derivatives and copper (II) salts for promoting oxidation of catechol to o-quinone. Catalysts, 13(1), 162. https://doi.org/10.3390/catal13010162
You, P. Y., Mo, K. M., Wang, Y. M., Gao, Q., Lin, X. C., Lin, J. T., Li, D. (2024). Reversible modulation of interlayer stacking in 2D copper-organic frameworks for tailoring porosity and photocatalytic activity. Nature Communications, 15(1), 194. https://doi.org/10.1038/s41467-023-44552-w
Shi, K., Mathivathanan, L., Herchel, R., Boudalis, A. K., Raptis, R. G. (2020). Supramolecular Assemblies of Trinuclear Copper (II)-Pyrazolato Units: A Structural, Magnetic and EPR Study. Chemistry, 2(3), 626–644. https://doi.org/10.3390/chemistry2030039
Davydenko, Y. M., Vynohradov, O. S., Pavlenko, V. O., Fesych, I. V., Fritsky, I. O., Naumova, D. D., Shova, S. (2024). [Synthesis and crystal structure of Copper (II) 9-Azametallacrowns-3 with 4-iodopyrazole]. Journal of Chemistry and Technologies, 32(3), 554–569. (in Ukrainian). https://doi.org/10.15421/jchemtech.v32i3.305413
Vynohradov, O. S., Davydenko, Y. M., Pavlenko, V. O., Naumova, D. D., Fritsky, I. O., Shova, S., Prysiazhna, O. V. (2023). CuBr2 as a bromination agent of pyrazole-based ligand: synthesis of copper (II) coordination compounds by oxidative dissolution of copper powder in organic solvents. Journal of Chemistry and Technologies, 31(3), 493–506. https://doi.org/10.15421/jchemtech.v31i3.281190
Davydenko, Y. M., Vitske, V. A., Pavlenko, V. A., Haukka, M., Vynohradov, O. S., Fritsky, I. O. (2022). Synthesis, crystal structure and properties of coordination polymers based on (3, 5-dimethyl-1Н-pyrazole-4-yl)-acetic acid. Journal of Chemistry and Technologies, 30(2), 174–183. https://doi.org/10.15421/jchemtech.v30i2.252517
Mukherjee, R. (2000). Coordination chemistry with pyrazole-based chelating ligands: molecular structural aspects. Coordination Chemistry Reviews, 203(1), 151–218. http://dx.doi.org/10.1016/S0010-8545(99)00144-7
Senko, M. W. IsoPro Isotopic Abundance Simulator, v. 2.1; National High Magnetic Field Laboratory, Los Alamos National Laboratory: Los Alamos, NM, 1994.
CrysAlis PRO 1.171.43.144a (Rigaku OD, 2024), SHELXT 2018/2 (Sheldrick, 2018), SHELXL 2018/3 (Sheldrick, 2015), Olex2 1.5-ac6-020 (Dolomanov et al., 2009).
Groom, C. R., Bruno, I. J., Lightfoot, M. P., Ward, S. C. (2016). The Cambridge structural database. Structural Science, 72(2), 171–179.
Nakamoto, K. (2009). Infrared and Raman Spectra of Inorganic and Coordination Compounds. Hoboken, New Jersey: John Wiley&Sons.
Chow, Y. M., Britton, D. O. Y. L. E. (1975). The crystal structures of dimethylthallium cyanide, azide, cyanate, and thiocyanate. Structural Science, 31(7), 1922–1929. https://doi.org/10.1107/S0567740875006486
Mezei, G., Zaleski, C. M., Pecoraro, V. L. (2007). Structural and functional evolution of metallacrowns. Chemical reviews, 107(11), 4933–5003. https://doi:10.1021/cr078200h
Addison, A. W., Rao, N. T., Reedijk, J., van Rijn, J., Verschoor, G. C. (1984). Synthesis, structure, and spectroscopic properties of copper(II) compounds containing nitrogen–sulphur donor ligands; the crystal and molecular structure of aqua[1,7-bis(N-methylbenzimidazol-2′-yl)-2,6-dithiaheptane]copper(II) perchlorate. J. Chem. Soc., Dalton Trans. 1349–1356. https://doi:10.1039/dt9840001349.
Yang, L., Powell, D. R., Houser, R. P. (2007). Structural variation in copper(I) complexes with pyridylmethylamide ligands: structural analysis with a new four-coordinate geometry index, τ4. Dalton Trans. (9), 955–64. https://doi:10.1039/b617136b. PMID 17308676
Spackman, P. R., Turner, M. J., McKinnon, J. J., Wolff, S. K., Grimwood, D. J., Jayatilaka, D., Spackman, M. A. (2021). CrystalExplorer: a program for Hirshfeld surface analysis, visualization and quantitative analysis of molecular crystals. Applied Crystallography, 54(3), 1006–1011
Downloads
Published
Issue
Section
License
Copyright (c) 2025 Oles Honchar Dnipro National University

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

