STUDY OF THE La-Ni-Ce TERNARY SYSTEM ACROSS THE LaNi5-CeNi5, LaNi5-CeNi, AND LaNi-CeNi POLYTHERMAL SECTIONS
DOI:
https://doi.org/10.15421/jchemtech.v33i4.335435Keywords:
Transition metals, intermetallic compounds, LaNi5, CeNi5, solid solution, phase diagram.Abstract
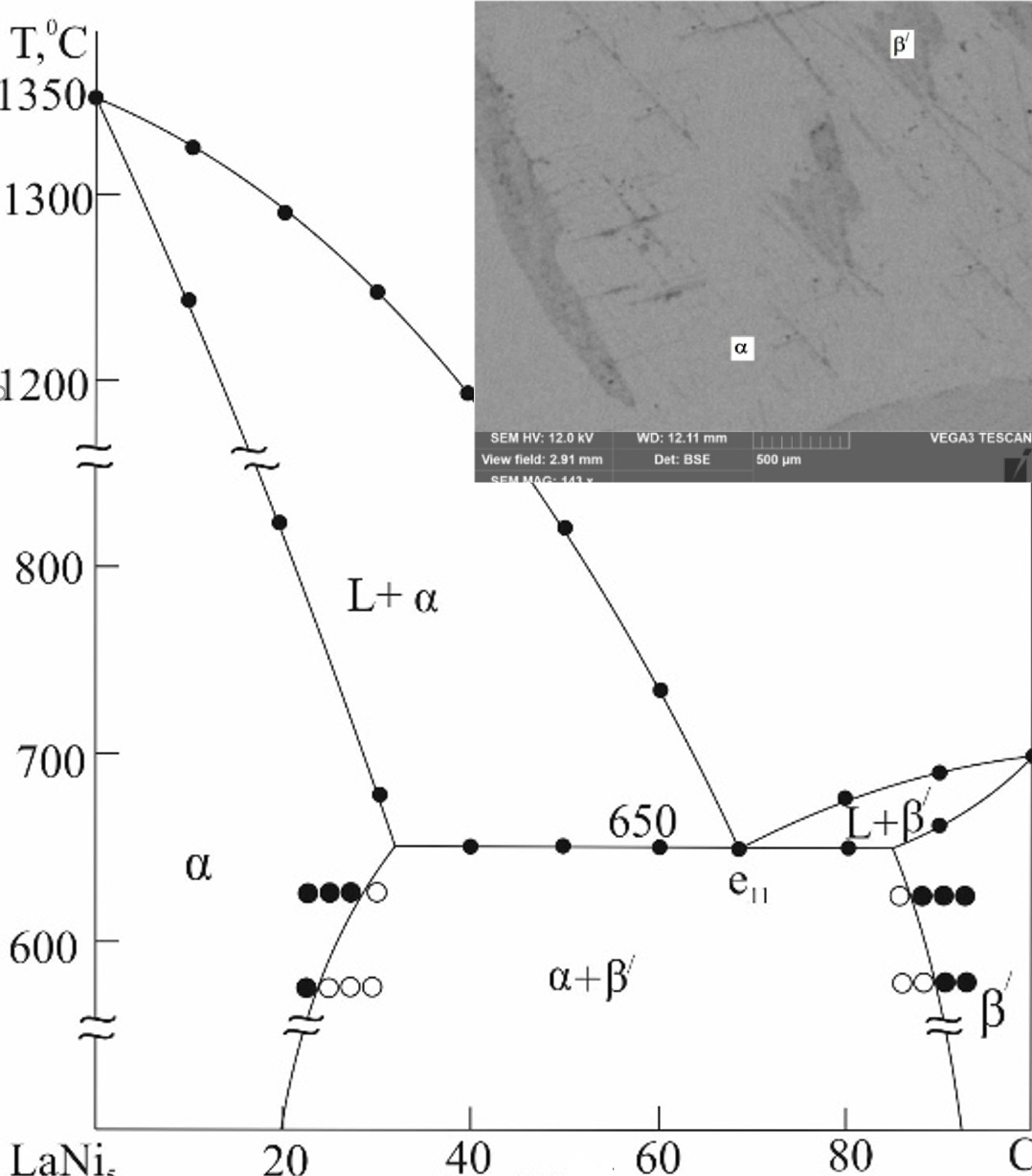
Intermetallic compounds of transition metals, especially lanthanides, and the new phases based on them attract the attention of researchers as effective adsorbers for hydrogen. Using physicochemical analysis techniques, including Differential Thermal Analysis (DTA), Powder x-ray Diffraction (PXRD) and Scanning Electron Microscope (SEM), the ternary La-Ni-Ce system was investigated across the LaNi5-CeNi5, LaNi5-CeNi, and LaNi-CeNi polythermal quasi-binary sections. The corresponding (T-x) phase diagrams have been constructed for the title system. The phase diagrams are quasi-binary and of the eutectic type. The systems have revealed wide regions of solid solutions based on starting compounds LaNi5, LaNi, CeNi5, CeNi. It has been established that the coordinates of the eutectic point formed on the basis of the initial components for the LaNi5-CeNi5, LaNi5-CeNi, and LaNi-CeNi sections are 50 mol% CeNi5, 1150 °C, 68 mol% CeNi, 650 °C, and 50 mol% CeNi, 620 °C, respectively. The obtained variable-composition phases can be considered as promising materials for hydrogen absorption.
References
Molinas, B., Pontarollo, A., Scapi, M., Peretti, H., Melnichu, M., Corso, H., Auror, A., Mirabile D., Montone, A. (2016). The optimization of MmNi5−xAlx hydrogen storage alloy for sea or lagoon navigation and transportation. Int. J. Hydrog. Energy. 41(32), 14484–14490. https://doi.org/10.1016/j.ijhydene.2016.05.222
Srivastava, S., Panwar, K. (2015). Effect of transition metals on ball-milled MmNi5 hydrogen storage alloy. Mater. Renew. Sustain. Energy. 4(19), 1–10. https://doi.org/10.1007/s40243-015-0062-9
Kulova, T. L., Nikolayev, I. I., Fateyev, V.N., Aliyev, A. Sh. (2018). Modern electrochemical systems of energy accumulation. Chem. Problems. 16(1), 9–34. https://doi.org/10.32737/2221-8688-2018-1-9-34
Todorovic, R. (2015). Hydrogen Storage Technologies for Transportation Application. J. Undergraduate Research. 8(1), 56–59. https://doi.org/ 10.5210/jur.v8i1.7541
Kurc, B., Gross, X., Szymlet, N., Rymaniak, Ł., Wozniak, K., Pigłowska, M. (2024). Hydrogen-powered vehicles: a paradigm shift in sustainable transportation. Energies, 17(69), 4768. https://doi.org/ 10.3390/en17194768
Barthelemy, H., Weber, M., Barbier, F. (2017). Hydrogen storage: Recent improvements and industrial perspectives. Int. J. Hydrogen Energy, 42(11), 7254–7262. https://doi.org/10.1016/j.ijhydene.2016.03.178
Talaganis, B.A. Esquivel, M.R. Meyer, G. (2009). Atwo-stage hydrogen compressor based on (La, Ce, Nd, Pr)Ni5 intermetallics obtained by lowen ergyme chanical alloying–low temperature annealing treatment. Int. J. Hydrogen Energy, 34, 2062–2068. https://doi.org/10.1016/j.ijhydene.2008.11.052
Ngameni, R. Mbemba, N. Grigoriev, S. A., Millet, P. (2011). Comparative analysis of the hydriding kinetics of LaNi5, La0.8Nd0.2Ni5 and La0.7Ce0.3Ni5 compounds. Int.J. Hydrogen Energy, 36(6), 4178–4184. https://doi.org/10.1016/j.ijhydene.2010.06.107
Lototskyy, M.V., Yartys, V.A. Pollet, B.G., Jr Bowman, R. C. (2014). Metal hydride hydrogen compressors: A review. Int. J. Hydrog Energy, 39(11), 5818–5851. https://doi.org/10.1016/j.ijhydene.2014.01.158
Gahleitner, G. (2013). Hydrogen from renewable electricity: an international review of power-to-gas pilot plants for stationary applications. Int. J. Hydrog Energy, 38(5), 2039–2061. https://doi.org/10.1016/j.ijhydene.2012.12.010
An, X.H., Gu, Q.F., Zhang, J.Y., Chena, S.L., Yu, X.B., Li, Q. (2013). Experimental investigation and thermodynamic reassessment of La–Ni and LaNi5–H systems. Calphad, 40, 48–55. https://doi.org/ 10.1016/j.calphad.2012.12.002
Afshari, M. (2017). Structural and magnetic properties of LaNi5 and LaNi3.94Al1.06 alloys, before and after hydrogenation. J. Supercond. Nov. Magn,30 (8),2255–2259. https://doi.org/10.1007/s10948-017-4045-1
Sato, T., Saitoh, H., Utsumi, R., Ito, J., Nakahira, Y., Obana, K., Takagi, S., Orimo, S. (2023). Hydrogen Absorption Reactions of Hydrogen Storage Alloy LaNi5 under High Pressure. Molecules, 28, 1256. https://doi.org/10.3390/molecules28031256
Mardani, M., Bajenova, I., Khvan, A., Cheverikin, V., Richter, K. (2020). Phase transformations and phase equilibria in the LaeNi and LaeNieFe systems. Part 1: Liquidus & solidus projections. J. Alloys and Compd. 845(10), 156356. DOI:10.1016/j.jallcom.2020.156356
Joubert, J.-M., Paul-Boncour, V., Cuevas, F., Zhang, J., Latroche, M. (2021). LaNi5 Related AB5 Compounds: Structure, Properties and Applications. J. Alloys Compd. 862, 158163. https://doi.org/10.1016/j.jallcom.2020.158163.
Zareii, S. M., Arabi, H., Pourarian, F. (2014). A comprehensive investigation of structural, morphological, hydrogen absorption and magnetic properties of MmNi4.22Co0.48Mn0.15Al0.15 alloy. Int. J. Mod. Phys. B, 28(19), 1450125. https://doi.org/10.1142/S0217979214501252
Zhang, L., Allendorf, M.D., Balderas–Xicohténcatl, R., Broom, D.P., Fanourgakis, G.S., Froudakis, G.E. (2022). Fundamentals of Hydrogen Storage in Nanoporous Materials. Prog. Energy., 4(4), 042013. doi:10.1088/2516-1083/ac8d44
Shashikala, K. (2012). 15-Hydrogen storage materials. İn book, Functional materials. London: Elsevier. https://doi.org/10.1016/B978-0-12-385142-0.00015-5
Liu, Y., Chabane, D., Elkedim, O. (2024). Optimization of LaNi5 hydrogen storage properties by the combination of mechanical alloying and element substitution. Int. J. Hydrogen Energy. 53(69), 394–402. doi:10.1016/j.ijhydene.2023.12.038
Wen, X., Wang, B., Li, C., Liu, T. (2024). Effect of La doping on the structural stability and hydrogen adsorption behavior of Ce-La alloys. J. Alloys Compd. 982, 173553. https://doi.org/10.1016/j.jallcom.2024.173553
Jain, R.K., Jain, A., Agarwal, Sh. N., Lalla, P., Ganesan, V., Phase, D.M., Jain, I.P. (2007). Hydrogenation behaviour of Ce-based AB5 intermetallic compounds. J. Alloys Compd., 440, 84–88. https://doi.org/10.1016/j.jallcom.2006.08.326
Borzone, E. M., Baruj, A., Blanco, M.V., Meyer, G.O. (2013). Dynamic measurements of hydrogen reaction with LaNi5-xSnx alloys. Int. J. Hydrogen Energy, 38(18), 7335–7343. https://doi.org/10.1016/j.ijhydene.2013.04.035
Jain, R.K., Jain, A., Agarwa Sh., Lalla, N.P., Ganesan, V., Phase, D.M., Jain, I.P. (2007). Characterization and hydrogenation of CeN 5-x Crx (x=0, 1, 2) alloys. J. Alloys Compd., 430, 165–169. https://doi.org/10.1016/j.jallcom.2006.05.013
Bakhtiyarli, I.B, Mammadov, V.S, Mukhtarova, Z.M, Abdullayeva, A.S (2023). Phase Equilibrium In The Quasi-Ternary System Y2O2S–Ga2S3–Tb2O2S. Azerb. Chem. Journal, 1, 55–64.
Mammadov, F. M. (2021). New version of the phase diagram of the MnTe-Ga2Te3 system. New Materials, Compounds and Applications, 5(2), 116–121.
Mammadov, F.M., Babanly, D.M., Orujlu, E.N., Niftiyev, N.N., Salmanov, F.T., Gasimov, R. J., Bayramov, M.A., Amiraslanov, I. R., Babanly, M. B. (2025). The phase equilibria in the MnSe–Ga2Se3–In2Se3 system, crystal structure and some physical properties of MnGaInSe4. J. Alloys Compd., 1036, 181814. https://doi.org/10.1016/j.jallcom.2025.181814
Mammadov, Sh.H., Ismailova, R. A., Gasanova, M. B. (2025). Ag2S-Ga2S3-PbS quasi-ternary system. Journal of chemistry and technologies. 33(2), 1-7.
Ismayilova, E.N., Baladzhayeva, A.N., Mashadiyeva, L.F. (2021). Phase equilibria along the Cu3SbSe4-GeSe2 section of the Cu-Ge-Sb-Se system. New Materials, Compounds and Applications, 5(1), 52–58.
Aghazade, A. I., Orujlu, E. N., Salimov, Z. E., Mammadov, A. N., Babanly, M. B. (2023). Experimental investigation of the solid phase equilibria at 300 K in the SnBi2Te4-PbBi2Te4-Bi2Te3 system. Phys. Chem. Solid State, 24(3), 453–459. https://doi.org/10.15330/pcss.24.3.453-459
Mammadov, F.M. (2020). FeS-FeGa2S4-FeGaInS4 system. Chem. Problems, 2(18), 214–221. https://doi.org/10.32737/2221-8688-2020-2-214-221
Ismailova, E. N. Mashadieva, L. F., Bakhtiyarly, I. B., Gasymov, V. A., Gurbanova, R. J., Mammadova, F. M. (2025). Phase Equilibria In The Cu2Se - Cu3SbSe4 - Cu2SnSe3 System. Chem. Problems, 1(23), 36–46. https://doi.org/10.32737/2221-8688-2025-1-36-46
Zhang, D., Jinke, T., Gschneidner, K. A. (1991). Are determination of the La–Ni phase diagram from LaNi to LaNi5 (50–83.3 at%Ni). J. Less- Common Met., 169, 45–53. https://doi.org/10.1016/0022-5088(91)90234-U
Inui, H., Yamamoto, T., Zhang, D., Yamaguchi, M. (1999). Microstructures and defect structures in intermetallic compounds in the La–Ni alloy system. J. Alloys Compd., 293, 140–145. https://doi.org/10.1016/S0925-8388(99)00314-X
An, X.H., Gu, Q.F., Zhang, J.Y., Chen, S.L., Yu, X.B., Li, Q. (2013). Experimental investigation and thermodynamic reassessment of La-Ni and LaNi5–H systems. Calphad, 40, 48–55. https://doi.org/10.1016/j.calphad.2012.12.002
Okamoto, H. (2020). Supplemental literature review of binary phase diagrams: Al-Pt, As-U, C-Li, C-Mg, Cd-Nd, Co-Ta, Fe-Re, Ga-Y, La-Ni, O-V, P-Si, and Re-Zr. J. Phase Equilib. Diffus. 41, 722–733. https://doi.org/10.1007/s11669-020-00839-9
Dwight, A.E., Conner, Jr, R.A., Downey, J.W. (1965). Equiatomic compounds of the transition and lanthanide elements with Rh, Ir, Ni and Pt. Acta Crystallogr., 18 (5), 835–839. https://doi.org/10.1107/S0365110X65002050
Xiong, W. Du, Y. Lu, X. Schuster, J., Chen, H. (2007). Reassessment of the Ce-Ni binary system supported by key experiments and ab initio calculations. Intermetallics, 15(11), 1401–1408. https://doi.org/10.1016/j.intermet.2007.04.004
Pourarian, F., Wallace, W.E. (1982). Hydrogen storage in CeNi5-xCux. Less-Common Metals, 87(2), 275–281. https://doi.org/10.1016/0022-5088(82)90094-7
Downloads
Published
Issue
Section
License
Copyright (c) 2025 Oles Honchar Dnipro National University

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

