PHASE EQUILIBRIA IN THE HfO2–La2O3–Nd2O3 SYSTEM AT 1500 °С
DOI:
https://doi.org/10.15421/jchemtech.v33i4.336018Keywords:
Phase diagram, isothermal section, hafnium dioxide, rare-earth oxides, HfO2–La2O3–Nd2O3 system, solid solutionsAbstract
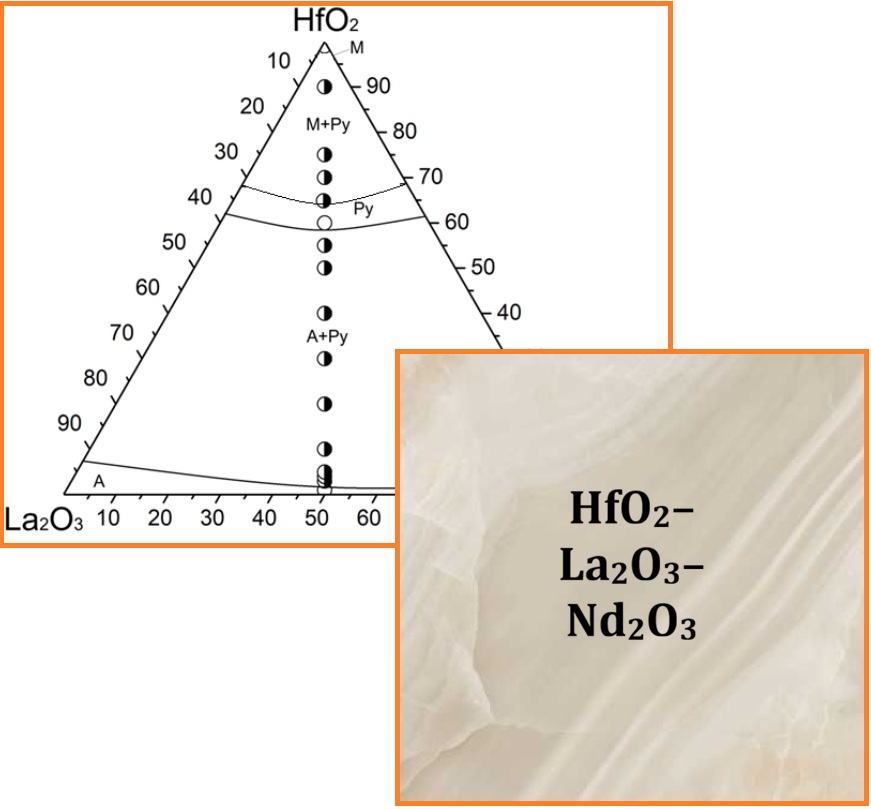
Phase equilibria within the HfO2–La2O3–Nd2O3 ternary system (across the 0-100 mol% HfO2 concentration range) were investigated using X-ray phase analysis and microstructural analysis. Based on the experimental results, an isothermal section of the phase diagram at 1500 °С was constructed. It was established that at this temperature, the HfO2–La2O3–Nd2O3 system exhibits three continuous solid solution series: the hexagonal A-Ln2O3 type (P63/m), the monoclinic M-HfO2 type (P21/c), and an ordered pyrochlore-type phase (Fd3-m), Ln2Hf2O7. The corresponding homogeneity regions border the biphasic fields (M + Py) and (Py + A), respectively. No new phases were found to form under the investigated conditions. The lattice parameter (a) of the ordered pyrochlore-type phase Ln2Hf2O7 varies linearly: from a = 1.0572 nm for a biphasic sample (A + Py) of composition 2 mol% HfO2 – 49 mol% La2O3 – 49 mol% Nd2O3, to a = 1.0696 nm for the solid solution boundary composition, and a = 1.06657 nm for a biphasic sample (Py + M) of composition 65 mol% HfO2 – 17.5 mol% La2O3 – 17.5 mol% Nd2O3.
References
Du, J., Liu, R.-J., Wan, F., Duan, L., Li, J., Wang, Y. (2022) Study on La2(Hf1-xTix)2O7 (x ≤ 0.20) pyrochlores for potential thermal/environmental barrier coating applications. Ceramics International, 48, 671–681. https://doi.org/10.1016/j.ceramint.2022.07.300
Matović, B., Belozerova, N., Kozlenko, D., Zel, Yu., Maletaškić, J., Zagorac, D., Butulija, S., Cvijović-Alagić, I. (2024) High-pressure behaviour of high-entropy A2B2O7 pyrochlore. Ceramics International, 50(24), 52649–52654. https://doi.org/10.1016j.ceramint.2024.10.116
Wang, Z., Zhu, Ch., Wang, H., Wang, M., Liu, Ch., Yang, D., Li, Yu. (2025) Preparation and irradiation stability of A2B2O7 pyrochlore high-entropy ceramic for immobilization of high-level nuclear waste. Journal of Nuclear Materials, 574, 154212. https://doi.org/10.1016/j.jnucmat.2022.154212
Eberman, K.W., Wuensch, B.J., Jorgensen, J.D. (2002). Order–disorder transformations induced by composition and temperature change in (SczYb1-z)2Ti2O7 pyrochlores, prospective fuel cell materials. Solid State Ionics, 148, 521–526. https://doi.org/10.1016/S0167-2738(02)00099-1
Vayer, F., Decorse, C., Bérardan. D., Dragoe, D., Dragoe, N., (2023) Investigation of the chemical versatility in high-entropy pyrochlores. Journal of the American Ceramic Society, 106(4), 2601–2621. https://doi.org/10.1111/jace.18922
Ji, Y., Jiang, D., Fen, T., Shi J., (2005). Fabrication of transparent La2Hf2O7 ceramics from combustion synthesized powders. Materials Research Bulletin, 40, 553–559. https://www.jim.org.cn/EN/10.3724/SP.J.1077.2011.00929
Wang, Z., Zhou, G., Jiang, D., Wang, S. (2018). Recent development of A2B2O7 system transparent ceramics. Journal of Advanced Ceramics, 7(4), 289–306. https://doi.org/10.1007/s40145-018-0287-z
Zou, X.Q., Zhou, G.H., Yi, H.L., Wang Sh., (2011). Fabrication of transparent Y2Hf2O7 ceramic from combustion synthesized powders. Journal of Inorganic Materials, 26, 929–932. https://www.jim.org.cn/EN/10.3724/SP.J.1077.2011.00929
Lehan, J.P., Mao, Y., Bovard, B.G., Macleod, H.A. (1991). Optical and microstructural properties of hafnium dioxide thin films. Thin Solid Films, 203(2), 227–250. https://doi.org/10.1016/0040-6090(91)90131-G
Nishide, T., Honda, S., Matsuura, M., Ide, M. (2000). Surface, structural and optical properties of sol-gel derived HfO2 films. Thin Solid Films, 371(1), 61–65. https://doi.org/10.1016/S0040-6090(00)01010-5
Cheynet, M.C., Pokrant, S., Tichelaar, F.D., Rouvìre, J.L. (2007). Crystal structure and band gap determination of HfO2 thin films. Journal of Applied Physics, 101(5), 054101. https://doi.org/10.1063/1.2697551
Lakiza, S., Hrechaniuk, M., Redko, V., Ruban, O., Tyshchenko, Ya., Makudera, O., Dudnik, O. (2021). [The role of hafnium in modern thermal barrier coatings]. Poroshkova metalurhiia 1/2, 99–109. (in Ukrainian). http://www.materials.kiev.ua/article/3195
McGinnity, T.L., Sokolova, V., Prymak, O., Roeder, R.K. (2021) Colloidal stability, cytotoxicity, and cellular uptake of HfO2 nanoparticles. Journal of Biomedical Materials Research Part B: Applied Biomaterials, 109(10), 1407–1417. https://doi.org/10.1002/jbm.b.34800
Siboro, P.Y., Kumar, A., Lai, P., Jayakumar, J., Mi, F., Chen, H., Chang, Y., Sung, H., (2024). Harnessing HfO2 nanoparticles for wearable tumor therapy and biomedical imaging. ACS Nano, 18(3), 2485–2499. https://doi.org/10.1021/acsnano.3c11346
Wang, X., Wang, D., Liao, Y., Guo, X., Song, Q., Liu, W., Gu, Ch., Du, Sh., Sun, B., Gu, Z. (2025) Hafnium oxide-based sensitizer with radiation-triggered cuproptosis for radiotherapy. Nanotoday, 61, 102626. https://doi.org/10.1016/j.nantod.2024.102626
Wang, J., Pan, J., Tang, Y., Chen, J., Fei, X., Xue, W., Liu, X. (2023) Advances of hafnium based nanomaterials for cancer theranostics. Frontiers in Chemistry, 11, 1283924.
https://doi.org/10.3389/fchem.2023.1283924
Shevchenko A., Lopato L., Zaitseva Z., (1984) Vzaimodeistvie HfO2 s oksidami lantana, prazcodima i neodima pri vysokikh temperaturakh. Neorganicheskie Materialy, 20(9), 1530–1534.
Andrievskaya E. (2010). Phase equilibriums in systems of hafnia, zirconia, and yttria with REE oxides. Monograph, Kyiv: Naukova Dumka.
Duran, P. (1975). Phase relationships in the systems HfO2-La2O3 and HfO2-Nd2O3. Ceramuria International, 1, 10–13. https://doi.org/10.1016/0390-5519(75)90032-0
Glushkova, V., Sozonova, L., Ganits, F. (1978). [System research Nd2O3–HfO2]. Izvestiya AN SSSR. Neorganicheskie materialy, 14(1), 10. (in Russian).
Coutures, J., Sibieude, F., Foex, M. (1976). Etude a haute temperature des systems formes par les sesquioxides de lanthane avec les sesquioxydes de lanthanides II. Influence de la trempe sur la nature des phases obtenues a la temperature ambiante. Journal of Solid State Chemistry, 17(4), 377–384. https://doi.org/10.1016/S0022-4596(76)80006-0
Coutures, J., Rouanet, A., Verges, R., Foex, M., (1976). Etude a haute temperature des systems formes par le sesquioxyde de lanthane et les sesquioxydes de lanthanides. I. Diagrammes de phases (1400 °C < T < T liquide). Journal of Solid State Chemistry, 17(1–2), 172–182. https://doi.org/10.1016/0022-4596(76)90218-8
Zhu, L., Xie, Y., Wang, S., Xiao, Y. (2025). Lanthanum hydroxide showed best application potential in La-based materials based on its stable phosphate adsorption properties in complex water environments. Journal of Environmental Sciences, 152, 465–476. https://doi.org/10.1016/j.jes.2024.05.014
Gao, H., Zhang, D., Li, J., Liu, Q. (2023). Cyanometallate framework templated synthesis of hierarchically porous La(OH)3 for high-efficient and stable phosphorus removal from tailwater. Chemical Engineering Journal, 465, 142789. https://doi.org/10.1016/j.cej.2023.142789
Ismail W. et al., (2024) Investigating the physical and electrical properties of La2O3 via annealing of La(OH)3. Scientific Reports, 14(1), 7716. https://doi.org/10.1038/s41598-024-57848-8
Xi, W., Jian, P., Mao, X., Ning D., Xia X., Liu G., Shi D., Lan, Yu. (2021). Luminescent property of La(OH)3:Eu3+ nanorod and its decomposed compounds of LaOOH and La2O3. Materials Research Express, 105002. https://doi.org/10.1088/2053-1591/ac0eb6
Tian, H., Guo, J., Pei, Y., Hou, S., Wang, Y., Xi, Y. (2024). A synthetic method for lanthanum hydroxychloride suitable for industrialization and its thermal decomposition properties. RSC Advances, 14, 29282–29292.
https://doi.org/10.1039/D4RA03987D
Zhao, Z., Li, Z., Wu, L., Song Ya., Razanajatovo M., Sun Q., Jiao T., Peng Q., Zhang Q., (2023). Rational design of the nanocomposite by in-situ sub-10 nm La(OH)3 formation for selective phosphorus removal in waters. Separation and Purification Technology, 123306. https://doi.org/10.1016/j.seppur.2023.123306
Zhang, Y., Qian, Y., Li, W., Gao, X., Pan, B. (2019). Fluoride uptake by three lanthanum based nanomaterials: Behavior and mechanism dependent upon lanthanum species. Science of the Total Environment, 683, 609–616. https://doi.org/10.1016/j.scitotenv.2019.05.185
Downloads
Published
Issue
Section
License
Copyright (c) 2025 Oles Honchar Dnipro National University

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

