SYNTHESIS OF NOVEL N-BENZYL AND RELATED 1H-1,2,3-TRIAZOLE-4-CARBOXAMIDES AND THEIR ANTIBACTERIAL AND ANTIFUNGAL ACTIVITIES
DOI:
https://doi.org/10.15421/jchemtech.v33i4.336132Keywords:
1H-1,2,3-triazole-4-carboxamides, antimicrobial actionAbstract
A series of novel N-benzyl and related 1H-1,2,3-triazole-4-carboxamides was synthesized and investigated as potential antibacterial and antifungal agents. The new amides were obtained via a convenient synthetic route involving the cyclocondensation of aryl azides with β-ketoesters to form 1H-1,2,3-triazole-4-carboxylic acids, followed by their conversion into amides through the reaction of the corresponding acid chlorides with appropriate amines. A preliminary antimicrobial screening at a concentration of 32 µg/mL was conducted against a panel of bacterial strains, including Staphylococcus aureus, Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, and Acinetobacter baumannii, as well as fungal pathogens Candida albicans and Cryptococcus neoformans. Several compounds demonstrated selective or broad-spectrum inhibitory activity, with compound 5m showing the most consistent effectiveness across all tested strains. Notable antibacterial activity was observed for compound 5n (20.20 % inhibition of A. baumannii), while compounds 5a (22.35 % inhibition of C. neoformans) and 5h (17.70 % inhibition of C. albicans) exhibited pronounced antifungal activity. Compound 7a showed a promising dual action profile, inhibiting A. baumannii by 21.05 % and C. albicans by 13.20 %. Among all the tested microorganisms, A. baumannii and C. albicans were the most sensitive to the studied compounds. The results indicate that specific structural features of these 1,2,3-triazole-4-carboxamides contribute significantly to their biological activity and highlight their potential as scaffolds for the development of new antimicrobial agents.
References
Ho, C. S., Wong, C. T., Aung, T. T., Lakshminarayanan, R., Mehta, J. S., Rauz, S., Ting, D. S. (2025). Antimicrobial resistance: a concise update. Lancet Microbe, 6(1).
https://doi.org/10.1016/j.lanmic.2024.07.010
Jahromi, A. S., Namavari, N., Jokar, M., et al. (2025). Global knowledge, attitudes, and practices towards antimicrobial resistance among healthcare workers: a systematic review and meta-analysis. Antimicrob. Resist. Infect. Control, 14, 47.
https://doi.org/10.1186/s13756-025-01562-1
Oliveira, M., Antunes, W., Mota, S., Madureira-Carvalho, Á., Dinis-Oliveira, R. J., da Silva, D. D. (2024). An overview of the recent advances in antimicrobial resistance. Microorganisms, 12(9), 1920.
https://doi.org/10.3390/microorganisms12091920
Gilham, E. L., Pearce-Smith, N., Carter, V., et al. (2024). Assessment of global antimicrobial resistance campaigns conducted to improve public awareness and antimicrobial use behaviours: a rapid systematic review. BMC Public Health, 24, 396.
https://doi.org/10.1186/s12889-024-17766-w
Ljungquist, O., Nazarchuk, O., Kahlmeter, G., et al. (2023). Highly multidrug-resistant Gram-negative bacterial infections in war victims in Ukraine, 2022. Lancet Infect. Dis., 23(7), 784–786.
https://doi.org/10.1016/S1473-3099(23)00291-8
Plantinga, N. L., van Asten, S. A., Schijffelen, M. J., et al. (2025). Healthcare professionals challenged by 14 distinct carbapenemase-producing micro-organisms in a war-injured Ukrainian patient. Infection.
https://doi.org/10.1007/s15010-025-02523-x
Pallett, S. J., Morkowska, A., Woolley, S. D., et al. (2025). Evolving antimicrobial resistance of extensively drug-resistant Gram-negative severe infections associated with conflict wounds in Ukraine: an observational study. Lancet Reg. Health Eur., 52, 101274.
https://doi.org/10.1016/j.lanepe.2025.101274
Salma, U., Ahmad, S., Alam, M. Z., Khan, S. A. (2024). A review: Synthetic approaches and biological applications of triazole derivatives. J. Mol. Struct., 1301, 137240.
Abi, A. K., Sravani, S., Gujjarappa, R., et al. (2022). An overview on biological activities of 1,2,3-triazole derivatives. Nanostructured Biomaterials: Basic Structures and Applications, 401–423. https://doi.org/10.1007/978-981-16-8399-2_11
Kumar, S., Khokra, S. L., Yadav, A. (2021). Triazole analogues as potential pharmacological agents: A brief review. Future J. Pharm. Sci., 7(1), 106. https://doi.org/10.1186/s43094-021-00241-3
Rani, S., Teotia, S., Nain, S. (2024). Recent advancements and biological activities of triazole derivatives: a short review. Pharm. Chem. J., 57(12), 1909–1917. https://doi.org/10.1007/s11094-024-03096-z
Tian, G., Song, Q., Liu, Z., Guo, J., Cao, S., Long, S. (2023). Recent advances in 1,2,3 and 1,2,4 triazole hybrids as antimicrobials and their SAR: A critical review. Eur. J. Med. Chem., 259, 115603.
https://doi.org/10.1016/j.ejmech.2023.115603
Marzi, M., Farjam, M., Kazeminejad, Z., Shiroudi, A., Kouhpayeh, A., Zarenezhad, E. (2022). A recent overview of 1,2,3 triazole containing hybrids as novel antifungal agents: Focusing on synthesis, mechanism of action, and structure–activity relationship (SAR). J. Chem., 2022(1), 7884316.
https://doi.org/10.1155/2022/7884316
Poonia, N., Kumar, A., Kumar, V., Yadav, M., Lal, K. (2021). Recent progress in 1H 1,2,3 triazoles as potential antifungal agents. Curr. Top. Med. Chem., 21(23), 2109–2133.
http://dx.doi.org/10.2174/1568026621666210913122828
Pokhodylo, N., Finiuk, N., Klyuchivska, O., Tupychak, M. A., Matiychuk, V., Goreshnik, E., Stoika, R. (2022). Novel N-(4 thiocyanatophenyl)-1H-1,2,3-triazole-4-carboxamides exhibit selective cytotoxic activity at nanomolar doses towards human leukemic T-cells. Eur. J. Med. Chem., 241, 114633.
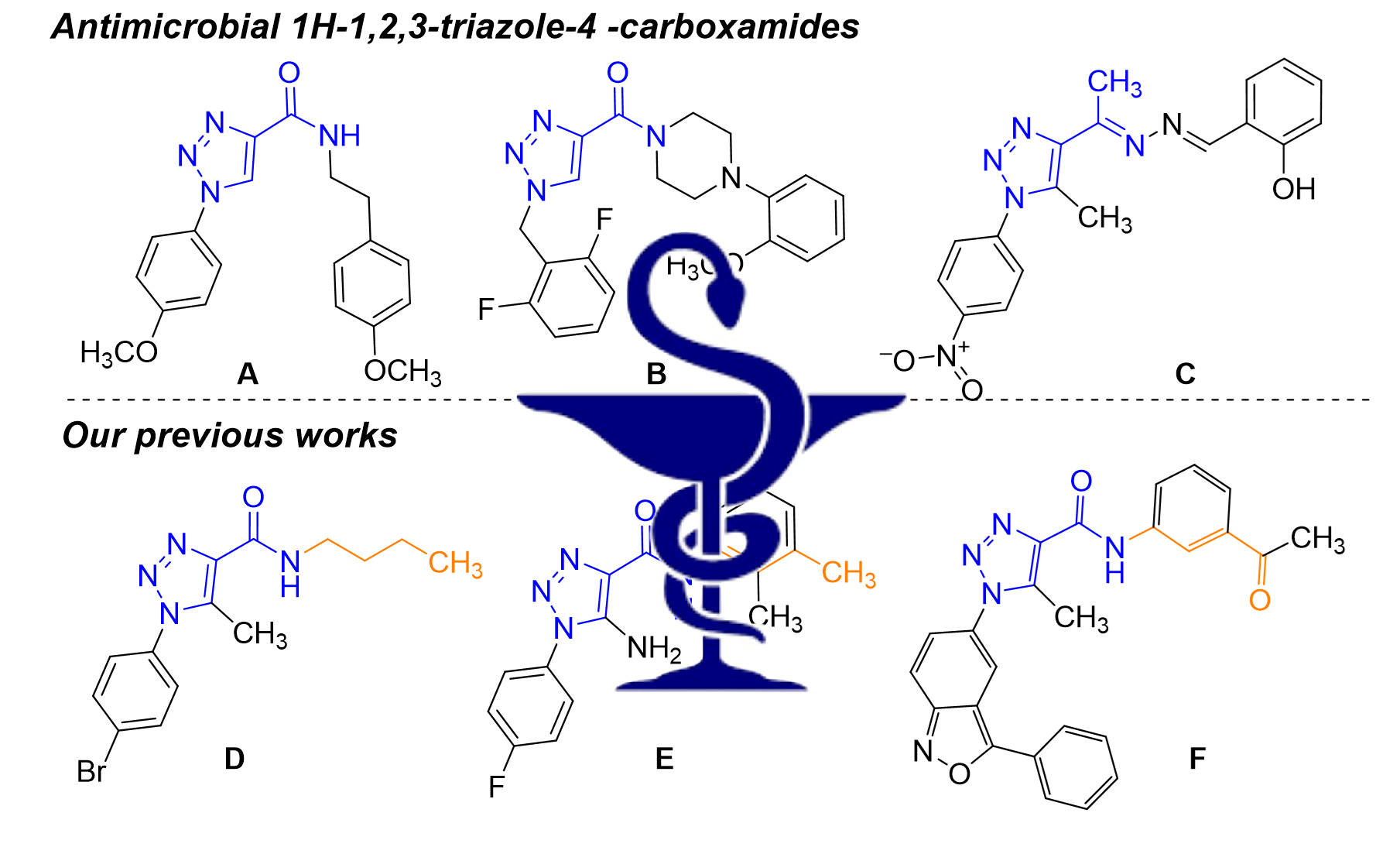
Pokhodylo, N., Manko, N., Finiuk, N., Klyuchivska, O., Matiychuk, V., Obushak, M., Stoika, R. (2021). Primary discovery of 1 aryl 5 substituted 1H 1,2,3 triazole 4 carboxamides as promising antimicrobial agents. J. Mol. Struct., 1246, 131146.
https://doi.org/10.1016/j.molstruc.2021.131146
Wang, Z. J., Gao, Y., Hou, Y. L., Zhang, C., Yu, S. J., Bian, Q., Li, Z. M., Zhao, W. G. (2014). Design, synthesis, and fungicidal evaluation of a series of novel 5 methyl 1H 1,2,3 trizole 4 carboxyl amide and ester analogues. Eur. J. Med. Chem., 86, 87–94.
Jadhav, R. P., Raundal, H. N., Patil, A. A., Bobade, V. D. (2017). Synthesis and biological evaluation of a series of 1,4 disubstituted 1,2,3 triazole derivatives as possible antimicrobial agents. J. Saudi Chem. Soc., 21(2), 152–159.
https://doi.org/10.1016/j.jscs.2015.03.003
Othman, E. M., Othman, D. I., Bayoumi, W. A., Habib, E.S.E., El Messery, S. M. (2025). New Azine Tagged 1,4,5 Trisubstituted 1,2,3 Triazoles Targeting Microbial Species Using Synthetic, Mechanistic, and In Silico Approach. J. Mol. Struct., 143282.
https://doi.org/10.1016/j.molstruc.2025.143282
Pokhodylo, N. T., Tupychak, M. A., Bonetskyi, O. O., Grynchyshyn, N. M., Matiychuk, V. S. (2024). Synthesis of Novel 1 (3 Phenylbenzo[c]isoxazol 5 yl)-1H-1,2,3-triazole-4-carboxamides and Their Antibacterial and Antifungal Activities. J. Chem. Technol., 32(2), 284–293.
https://doi.org/10.15421/jchemtech.v32i2.297699
Pokhodylo, N. T., Shyyka, O. Ya., Matiychuk, V. S., Obushak, M. D., Pavlyuk, V. V. (2017). A Novel Base Solvent Controlled Chemoselective Azide Attack on an Ester Group versus Keto in Alkyl 3 Substituted 3 Oxopropanoates: Mechanistic Insights. Chemistry Select, 2(21), 5871–5876.
https://doi.org/10.1002/slct.201700577
Pokhodylo, N. T., Shyyka, O. Ya., Goreshnik, E. A., Obushak, M. D. (2020). 4 Phosphonated or 4 Free 1,2,3 Triazoles: What Controls the Dimroth Reaction of Arylazides with 2 Oxopropylphosphonates? ChemistrySelect, 5(1), 260–264.
https://doi.org/10.1002/slct.201904688
Pokhodylo, N. T., Shyyka, O. Ya., Obushak, M. D. (2018). Convenient synthetic path to ethyl 1 aryl 5 formyl 1H 1,2,3 triazole 4 carboxylates and 1 aryl 1,5 dihydro 4H [1,2,3]triazolo[4,5 d]pyridazin 4 ones. Chem. Heterocycl. Compd., 54(8), 773–779.
https://doi.org/10.1007/s10593-018-2348-1
Pokhodylo, N. T., Matiychuk, V. S., Obushak, M. D. (2010). Synthesis of isothiocoumarin derivatives. Chem. Heterocycl. Compd., 46(2), 140–145.
https://doi.org/10.1007/s10593-010-0484-3
Pokhodylo, N. T., Shyyka, O. Ya., Obushak, M. D. (2014). Facile and efficient one pot procedure for thieno[2,3 e][1,2,3]triazolo[1,5 a]pyrimidines preparation. Synth. Commun., 44(7), 1002–1006.
https://doi.org/10.1080/00397911.2013.840729
Desselle, M. R., Neale, R., Hansford, K. A., Zuegg, J., Elliott, A. G., Cooper, M. A., Blaskovich, M. A. (2017). Institutional profile: Community for Open Antimicrobial Drug Discovery–crowdsourcing new antibiotics and antifungals. Future Sci. OA, 3(2), FSO171.
Downloads
Published
Issue
Section
License
Copyright (c) 2025 Oles Honchar Dnipro National University

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

