HARNESSING FERROCENECARBOXALDEHYDE IN MULTICOMPONENT REACTIONS FOR THE SYNTHESIS OF BIOACTIVE HETEROCYCLIC FERROCENES
DOI:
https://doi.org/10.15421/jchemtech.v33i3.336225Keywords:
condensation, heterocycles, ferrocenecarboxaldehyde, multicomponent reactions (MCR)Abstract
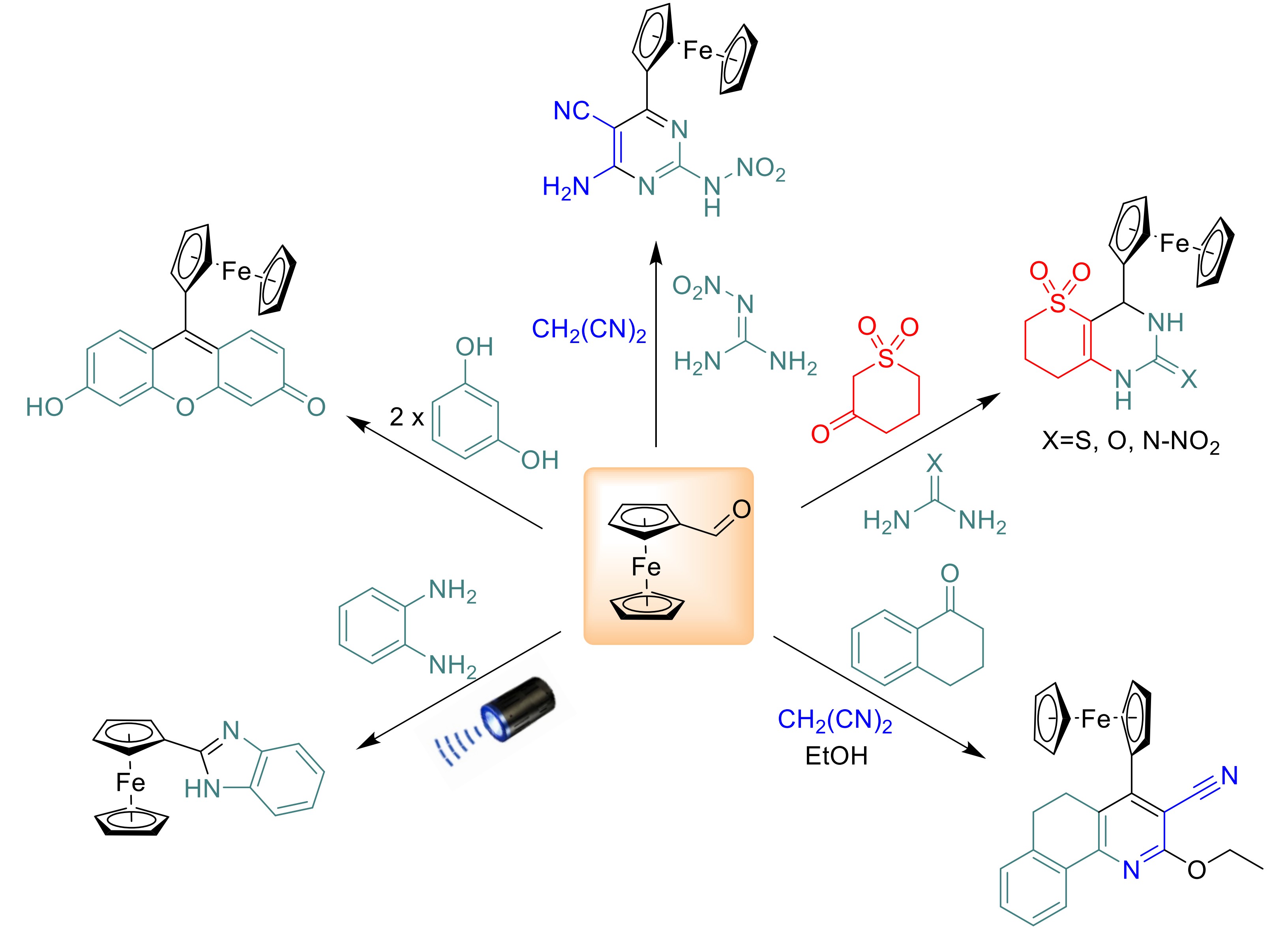
Multicomponent reactions (MCRs) offer a sustainable and efficient approach to synthesizing complex molecular scaffolds, aligning with green chemistry principles. This study explores the underexploited synthetic potential of ferrocenecarboxaldehyde in MCRs to construct heterocyclic ferrocene derivatives, motivated by their promising bioactivity in medicinal chemistry, potential in materials science, organic synthesis and environmental testing. Through reactions such as the Biginelli condensation, photocatalyzed synthesis, and tandem processes involving malononitrile, ketosulfone, and hydrazines, a diverse array of heterocyclic compounds was synthesized. Structural confirmation was achieved using ¹H and ¹³C NMR, GCMS, and LCMS, despite challenges with solubility and side reactions like decarboxylation and Cannizzaro processes. Successful syntheses included pyrimidine, pyridine and benzimidazole derivatives, with yields ranging from 5 % to 69 %. Several procedures were employed that avoided the need for column chromatography in target product isolation. These findings highlight ferrocenecarboxaldehyde versatility in generating bioactive heterocycles, paving the way for further exploration in drug discovery and materials science.
References
Mohlala, R. L., Rashamuse, T. J., Coyanis, E. M. (2024). Highlighting multicomponent reactions as an efficient and facile alternative route in the chemical synthesis of organic-based molecules: a tremendous growth in the past 5 years. Front. Chem., 12, 1469677. https://doi.org/10.3389/fchem.2024.1469677
Tymoshenko, K. I., Palchykov, V. A. (2024). Fused chiral azaheterocycles based on monosubstituted ferrocenes. Chem. Heterocycl. Compd., 60, 212–215. https://doi.org/10.1007/s10593-024-03322-2
Wani, W. A., Jameel, E., Baig, U., Mumtazuddin, S., Hun, L. T. (2015). Ferroquine and Its Derivatives: New Generation of Antimalarial Agents. Eur. J. Med. Chem., 101, 534–551. https://doi.org/10.1016/j.ejmech.2015.07.009
Parveen, H., Mukhtar, S., Azam, A. (2015). Novel ferrocenyl linked pyrazoline analogs as potent antiamoebic agents. J. Heterocycl. Chem., 53(2), 473–478. https://doi.org/10.1002/jhet.2427
Tirkey, V., Mishra, S., Dash, H. R., Das, S., Nayak, B. P., Mobin, S. M., Chatterjee, S. (2013). Synthesis, characterization and antibacterial studies of ferrocenyl and cymantrenyl hydrazone compounds. J. Organomet. Chem., 732, 122–129. https://doi.org/10.1016/j.jorganchem.2013.02.020
Ornelas, C., Astruc, D. (2023). Ferrocene-based drugs, delivery nanomaterials and Fenton mechanism: state of the art, recent developments and prospects. Pharmaceutics, 15(8), 2044. https://doi.org/10.3390/pharmaceutics15082044
Patra, M., Gasser, G. (2017). The medicinal chemistry of ferrocene and its derivatives. Nat. Rev. Chem., 1(9), 0066. https://doi.org/10.1038/s41570-017-0066
Wang, R., Chen, H., Yan, W., Zheng, M., Zhang, T., Zhang, Y. (2020). Ferrocene-containing hybrids as potential anticancer agents: current developments, mechanisms of action and structure–activity relationships. Eur. J. Med. Chem., 190, 112109. https://doi.org/10.1016/j.ejmech.2020.112109
Alaoui, N.-E. E., Boulhaoua, M., Hutai, D., Oláh-Szabó, R., Bősze, S., Hudecz, F., Csámpai, A. (2022). Synthetic and DFT modeling studies on Suzuki–Miyaura reactions of 4,5-dibromo-2-methylpyridazin-3(2H)-one with ferrocene boronates, accompanied by hydrodebromination and a novel bridge-forming annulation in vitro cytotoxic activity of the ferrocenyl–pyridazinone products. Catalysts, 12(6), 578. https://doi.org/10.3390/catal12060578
Csókás, D., Zupkó, I., Károlyi, B. I., Drahos, L., Holczbauer, T., Palló, A., Czugler, M., Csámpai, A. (2013). Synthesis, spectroscopy, X-ray analysis and in vitro antiproliferative effect of ferrocenylmethylene-hydrazinylpyridazin-3(2H)-ones and related ferroceno[d]pyridazin-1(2H)-ones. J. Organomet. Chem., 743, 130–138. https://doi.org/10.1016/j.jorganchem.2013.06.040
Csókás, D., Károlyi, B. I., Bősze, S., Szabó, I., Báti, G., Drahos, L., Csámpai, A. (2014). 2,3-Dihydroimidazo[1,2-b]ferroceno[d]pyridazines and a 3,4-dihydro-2H-pyrimido[1,2-b]ferroceno[d]pyridazine: synthesis, structure and in vitro antiproliferation activity on selected human cancer cell lines. J. Organomet. Chem., 750, 41–48. https://doi.org/10.1016/j.jorganchem.2013.10.057
Jernei, T., Bősze, S., Szabó, R., Hudecz, F., Majrik, K., Csámpai, A. (2017). N-Ferrocenylpyridazinones and new organic analogues: synthesis, cyclic voltammetry, DFT analysis and in vitro antiproliferative activity associated with ROS-generation. Tetrahedron, 73(43), 6181–6192. https://doi.org/10.1016/j.tet.2017.09.015
Kasyan, L. I., Sereda, S. V., Potekhin, K. A., Kasyan, A. O. (1997). Azabrendanes. I. Synthesis, structure and spectral parameters of N-(arylsulfonyl)-exo-2-hydroxy-4-azatricyclo[4.2.1.03,7]nonanes. Heteroatom Chem., 8(2), 177–184. https://doi.org/10.1002/(sici)1098-1071(1997)8:2<177::aid-hc10>3.0.co,2-o
Zefirov, N. S., Kasyan, L. I., Gnedenkov, L. Y., Shashkov, A. S., Cherepanova, E. G. (1979). Synthesis of norbornene endo-epoxide (3-oxatricyclo[3.2.1.02,4]octane). Tetrahedron Lett., 20(11), 949–950. https://doi.org/10.1016/s0040-4039(01)86058-5
Sviatenko, L., Kinney, C., Gorb, L., Hill, F. C., Bednar, A. J., Okovytyy, S., Leszczynski, J. (2014). Comprehensive investigations of kinetics of alkaline hydrolysis of TNT (2,4,6-trinitrotoluene), DNT (2,4-dinitrotoluene), and DNAN (2,4-dinitroanisole). Environ. Sci. Technol., 48(17), 10465–10474. https://doi.org/10.1021/es5026678
Hill, F. C., Sviatenko, L. K., Gorb, L., Okovytyy, S. I., Blaustein, G. S., Leszczynski, J. (2012). DFT M06-2X investigation of alkaline hydrolysis of nitroaromatic compounds. Chemosphere, 88(5), 635–643. https://doi.org/10.1016/j.chemosphere.2012.03.048
Tymoshenko, K. I., Shishkina, S. V., Palchykov, V. A. (2025). Ferrocene-containing tetrahydropyridazines via domino chemistry. J. Mol. Struct., 142145. https://doi.org/10.1016/j.molstruc.2025.142145
Dil, K. V., Palchykov, V. A. (2024). O,S,Se-containing Biginelli products based on cyclic β-ketosulfone and their postfunctionalization. Beilstein J. Org. Chem., 20, 2143–2151. https://doi.org/10.3762/bjoc.20.184
Xia, S., Yin, S., Tao, S., Shi, Y., Rong, L., Wei, X., Zong, Z. (2012). An efficient and facile synthesis of novel substituted pyrimidine derivatives: 4-amino-5-carbonitrile-2-nitroaminopyrimidine. Res. Chem. Intermed., 38(9), 2435–2442. https://doi.org/10.1007/s11164-012-0559-0
Ait Elmachkouri, Y., Irrou, E., Thiruvalluvar, A. A., Anouar, E. H., Varadharajan, V., Ouachtak, H., Mague, J.T., Sebbar, N. K., Essassi, E. M., Taha, M. L. (2023). Synthesis, crystal structure, spectroscopic characterization, DFT calculations, Hirshfeld surface analysis, molecular docking, and molecular dynamics simulation investigations of novel pyrazolopyranopyrimidine derivatives. J. Biomol. Struct. Dyn., 42(22), 12195–12213. https://doi.org/10.1080/07391102.2023.2268187
Mojtahedi, M. M., Hosseinkhany, S., Abaee, M. S., Mesbah, A. W. (2020). A divergent procedure for multicomponent synthesis of novel ferrocenyl derivatives of dicyanoanilines and cyanopyridines. Appl. Organomet. Chem., 34(8), e5675. https://doi.org/10.1002/aoc.5675
Monserrat, J.-P., Al-Safi, R. I., Tiwari, K. N., Quentin, L., Chabot, G. G., Vessières, A., Jaouen, G., Neamati, N., Hillard, E. A. (2011). Ferrocenyl chalcone difluoridoborates inhibit HIV-1 integrase and display low activity towards cancer and endothelial cells. Bioorg. Med. Chem. Lett., 21(20), 6195-6197. https://doi.org/10.1016/j.bmcl.2011.07.078
Guo, S., Wang, L., Tian, X., Zhaoa, Z., Wang, H. (2023). The crucial role of non-conjugated functional group on triplet manipulation of heterocycle aromaticity hot exciton materials. J. Mater. Chem. C., 11, 12511–12516. https://doi.org/10.1039/d3tc02831c
Siddiqui, I. R., Rai, P., Rahila, Srivastava, A., Shamim, S. (2014). Synthesis of imidazo[1,2-a]pyridine in the presence of iodine–water catalytic system. Tetrahedron Lett., 55(6), 1159–1163. https://doi.org/10.1016/j.tetlet.2013.12.088
Khosropour, A., Khodaei, M., Moghannian, H. (2005). A facile, simple and convenient method for the synthesis of 14-alkyl or aryl-14-H-dibenzo[a,j]xanthenes catalyzed by pTSA in solution and solvent-free conditions. Synlett, 2005(6), 955–958. https://doi.org/10.1055/s-2005-864837
Wu, W., Zhao, J., Wu, W., Chen, Y. (2012). Room temperature long-lived triplet excited state of fluorescein in N^N Pt(II) bisacetylide complex and its applications for triplet–triplet annihilation based upconversions. J. Organomet. Chem., 713, 189–196. https://doi.org/10.1016/j.jorganchem.2012.05.010
Santra, S., Mitra, S., Bagdi, A. K., Majee, A., Hajra, A. (2014). Iron(III)-catalyzed three-component domino strategy for the synthesis of imidazo[1,2-a]pyridines. Tetrahedron Lett., 55(37), 5151–5155. https://doi.org/10.1016/j.tetlet.2014.07.094
Sayyafi, M., Seyyedhamzeh, M., Khavasi, H. R., Bazgir, A. (2008). One-pot, three-component route to 2H-indazolo[2,1-b]phthalazine-triones. Tetrahedron, 64(10), 2375–2378. https://doi.org/10.1016/j.tet.2008.01.006
Dadwal, S., Kumar, M., Bhalla, V. (2020). “Metal-Free” nanoassemblies of AIEE-ICT-active pyrazine derivative: efficient photoredox system for the synthesis of benzimidazoles. J. Org. Chem., 85(21), 13906–13919. https://doi.org/10.1021/acs.joc.0c01965
Downloads
Published
Issue
Section
License
Copyright (c) 2025 Oles Honchar Dnipro National University

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

