PHASE RELATION IN THE Fe2O3-Gd2O3 SYSTEM AT 1300 AND 1400 °С
DOI:
https://doi.org/10.15421/jchemtech.v33i4.336812Keywords:
phase equilibria, iron oxide, gadolinium oxide, perovskite-type, solid solutions, lattice parameters.Abstract
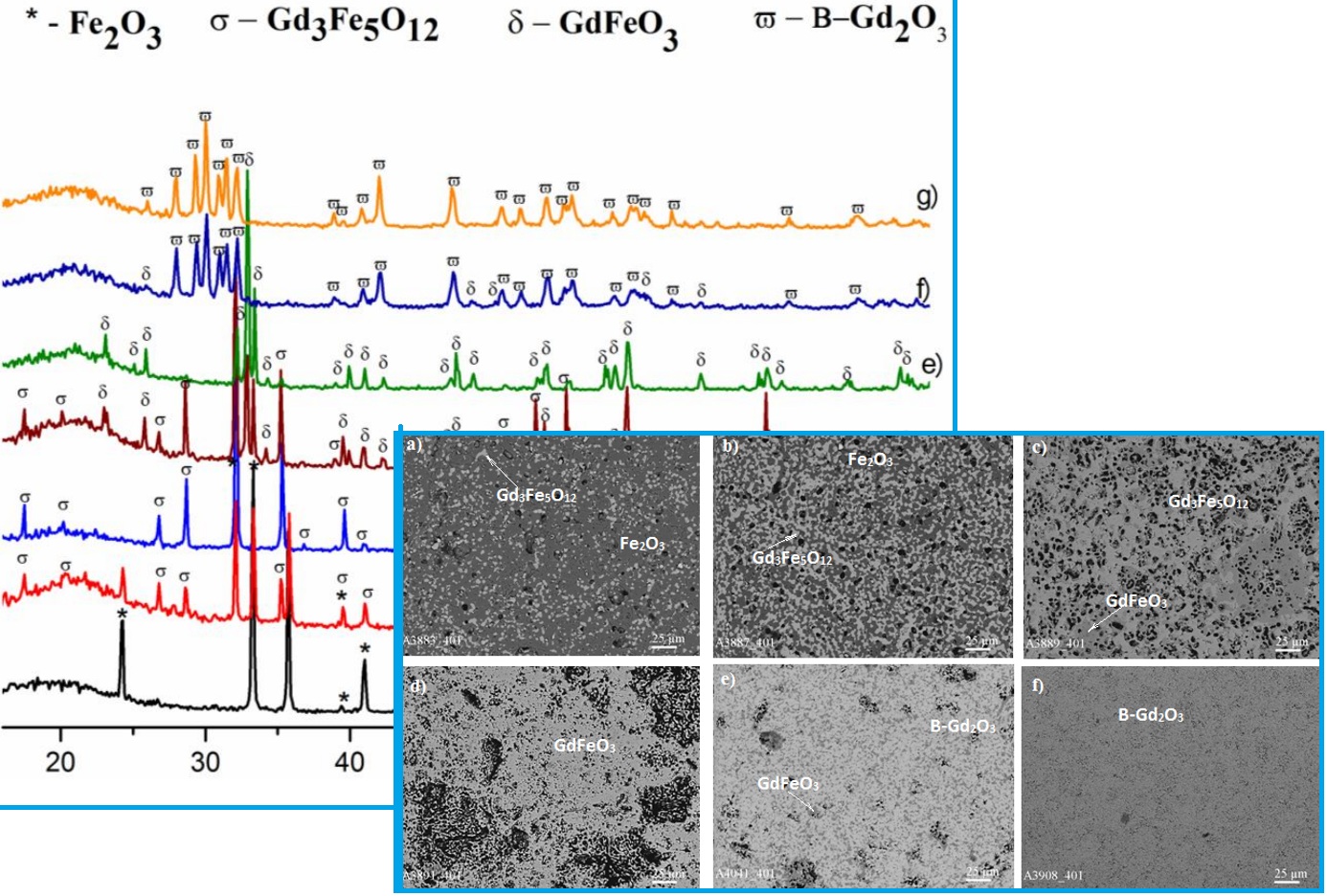
The phase interaction in the Fe2O3–Gd2O3 system at 1300 and 1400 °C were studied across the full concentration range using X-ray diffraction (XRD) and scanning electron microscopy (SEM). Samples, prepared in 1–5 mol% composition increments, were obtained by dissolving the oxides in HNO₃ (1 : 1), evaporating the solutions, and decomposing the nitrates at 800 °C for 2 h. The resulting powders were pressed at 10 MPa into 5 mm × 4 mm pellets and heat-treated in air at 1300 °C (300 h) and 1400 °C (100 h). The phase composition was determined using X-ray diffraction (XRD, DRON-3) and microstructural analysis (Superprobe-733, JEOL, Japan; Palo Alto, California, USA). At 1300 and 1400 °C, the isothermal sections of the Fe₂O₃–Gd₂O₃ phase diagram comprise four single-phase regions (B–Gd₂O₃, GdFeO₃(R), Gd₃Fe₅O₁₂, Fe₂O₃) and three two-phase regions (B–Gd₂O₃ + GdFeO₃, GdFeO₃ + Gd₃Fe₅O₁₂, Gd₃Fe₅O₁₂ + Fe₂O₃). The concentration dependence of the unit cell parameters for phases in the studied system was established. In the Fe₂O₃–Gd₂O₃ system at 1300 and 1400 °C, an ordered perovskite-type phase with orthorhombic distortion (GdFeO₃) is formed. Its homogeneity range at both temperatures extends from 48 to 52 mol.% Gd₂O₃.
References
Zhang, F., Huang, X., Qian, C., Zhu, L., Hida, N., Niu, G., Chen, X. (2012). Synergistic enhancement of iron oxide nanoparticle and gadolinium for dual-contrast MRI. Biochem. Biophys. Res. Commun., 425(4), 886–91. https://doi.org/10.1016/j.bbrc.2012.07.168
Loai, Y., Ganesh, T., Cheng, H.L.M. (2012) Concurrent Dual Contrast for Cellular Magnetic Resonance Imaging Using Gadolinium Oxide and Iron Oxide Nanoparticles. Int. J. Mol. Imaging., 230942. https://doi.org/10.1155/2012/230942
Zeng, Y., Wang, L., Zhou, Z., Wang, X., Zhang, Y., Wang, J., Mi, P., Liu, G., Zhou, L. (2017). Gadolinium hybrid iron oxide nanocomposites for dual T1- and T2-weighted MR imaging of cell labeling. Biomater. Sci., 5, 50–6. https://doi.org/10.1039/C6BM00706F
Khumaeni, A., Avicenna, S., Nurhasanah, I. (2024). Synthesis of iron oxide-gadolinium oxide nanocomposite produced by pulse laser ablation in carboxy methylcellulose as a contrast agent of magnetic resonance imaging. J. Mater. Sci. Mater. Electron., 35, 1422. https://doi.org/10.1007/s10854-024-13198-9
Montiel Schneider, M.G., Rivero, P.S., Muñoz Medina, G.A., Sanchez, F.H., Lassalle, V.L. (2023). Gd(OH)3 as Modifier of Iron Oxide Nanoparticles—Insights on the Synthesis, Characterization and Stability. Colloids Interfaces, 7(1), 8. https://doi.org/10.3390/colloids7010008
Pradhan, P.K., Panda, A.B., Mishra, G.K., Mohanty, N.K. (2025). Rare earth orthoferrites (RFeO3, R=rare earth elements): A comprehensive review of structural, dielectric, and magnetic properties. Smart. Mater. Manuf., 3, 100082. https://doi.org/10.1016/j.smmf.2025.100082
Shen, H., Zhao, Y., Li, L., Li, Q., Geng, H., Li, Y., Shen, X., Xu, J., Zhou, D., Tian, T., Ma, Y., Shang, J., Wu, A. (2024). Recent advances of rare earth iron garnet magneto-optical single crystals. J. Cryst Growth., 631, 127626. https://doi.org/10.1016/j.jcrysgro.2024.127626
Nakhaei, M., Bremholm, M., Khoshnoud, D.S. (2021). Structural and Magnetic Properties of RMO3 (R = Pr, Nd and M = Fe, Co) Perovskites. J. Supercond. Nov. Magn., 34, 3255–66. https://doi.org/10.1007/s10948-021-05992-x
Li, C.L., Zheng, S.S., Barasa, G.O., Zhao, Y.F., Wang, L., Wang, C.L., Lu, Y., Qiu, Y., Cheng, J.B., Luo, Y.S. (2021). A comparative study on magnetic behaviors and magnetocaloric effect in heavy rare-earth antiferromagnetic orthoferrites RFeO3 (R=Dy, Ho and Er). Ceram Int., 47(24), 35160–9. https://doi.org/10.1016/j.ceramint.2021.09.059
Chen, B., Katayama, T. (2023). Hexagonal RFeO3 (R = Dy, Er, and Lu) Films Grown on Glass Substrates with Both Magnetic and Ferroelectric Orders. ACS Appl Electron Mater., 5(1), 344–9. https://doi.org/10.1021/acsaelm.2c01356
Cho, E., Klyukin, K., Su, T., Kaczmarek, A., Ross, C.A. Composition-Dependent Ferroelectricity of LuFeO3 Orthoferrite Thin Films. Adv. Electron Mater., 9(3), 2300059. https://doi.org/10.1002/aelm.202300059
Pinho, S.L.C. (2018). Synthesis and Characterization of Rare-Earth Orthoferrite LnFeO3 Nanoparticles for Bioimaging. Eur. J. Inorg Chem., 468. https://doi.org/10.1002/ejic.201800468
Massa, N.E., Campo, L., Ta Phuoc, V., Alonso, J.A., Kayser, P. (2023). Low-temperature terahertz spectroscopy of LaFeO3, PrFeO3, ErFeO3, and LuFeO3: Quasimagnon resonances and ground-state multiplet transitions. Phys. Rev. B., 108, 115116. https://doi.org/10.1103/PhysRevB.108.115116
Weber, M., Guennou, M., Zhao, H.J., Iñiguez-González, J., Vilarinho, R., Kreisel, J. (2016). Raman spectroscopy of rare-earth orthoferrites RFeO3 (R=La, Sm, Eu, Gd, Tb, Dy). Phys. Rev. B., 94(21), 214103. https://doi.org/10.1103/PhysRevB.94.214103
Naseri, M.G., Tran, H.L.T., Nguyen, U.T., Nguyen, T.L. (2020). Sol-gel synthesis and the investigation of the properties of nanocrystalline holmium orthoferrite. Nanosyst. Phys. Chem. Math., 11(6), 698–704. https://doi.org/10.17586/2220-8054-2020-11-6-698-704
Chawda, N., Basu, M., Majumdar, D., Poddar, R., Mahapatra, S.K. (2019). Engineering of Gadolinium-Decorated Graphene Oxide Nanosheets for Multimodal Bioimaging and Drug Delivery. ACS Omega., 4(7),12470–9. https://doi.org/10.1021/acsomega.9b00883
Usman, M.S., Hussein, M.Z., Fakurazi, S., Ahmad Saad, F.F. (2017). Gadolinium-based layered double hydroxide and graphene oxide nano-carriers for magnetic resonance imaging and drug delivery. Chem. Cent. J., 11, 47. https://doi.org/10.1186/s13065-017-0275-3
Gwon, H., Kim, H., Lee, S. (2025). Gadolinium oxide-decorated graphene oxide-based dual-stimuli-responsive smart fluids. Nanoscale, 17, 5869–77. https://doi.org/10.1039/D4NR04941A
Zhu, X.H., Xiao, X.B., Chen, X.R., Liu, B.G. (2017). Electronic structure, magnetism and optical properties of orthorhombic GdFeO3 from first principles. RSC Adv., 7, 4054–61. https://doi.org/10.1039/C6RA25259A
Musić, S., Ilakovac, V., Ristić, M. (1992). Formation of oxide phases in the system Fe2O3−Gd2O3. J. Mater. Sci., 27, 1011–5. https://doi.org/10.1007/BF01197655
Musić, S., Popović, S., Czakó-Nagy, I., Gashi, F. (1993). Formation of oxide phases in the system Fe2O3−Gd2O3 Part II. J. Mater. Sci. Lett., 11, 869–73.
Buscaglia, V., Buscaglia, M.T., Giordano, L., Martinelli, A., Viviani, M., Bottino, C. (2002). Growth of ternary oxides in the Gd2O3–Fe2O3 system. A diffusion couple study. Solid State Ionics., 146(3–4), 257–71. https://doi.org/10.1016/S0167-2738(01)01021-9
Khan, M.S., Mukherje, A., Kumaresan, R. (2025). Electrochemical deoxidation behavior of Gd2O3 Fe2O3 oxides in LiCl and CaCl2 melts. J. Appl. Electrochem., 55, 1601–14. https://doi.org/10.1007/s10800-024-02256-z
Chudinovych, O. V., Vedel, D.V., Stasyuk, O.O., Tomila, T.V., Aguirre, M.H. (2024). Production, structure and magnetic properties of nanocomposites based on the perovskite phase LaFeO3. Solid State Sci., 157, 107699. https://doi.org/10.1016/j.solidstatesciences.2024.107699
Chudinovych, O. V., Vedel, D.V., Stasyuk, O.O., Samelyuk, A. V., Aguirre, M.H. (2024). Phase relations in the Nd2O3-Fe2O3 system: Structure andmagnetic properties of perovskite NdFeO3 ceramics. Process. Appl. Ceram., 18(3), 314–322.
Downloads
Published
Issue
Section
License
Copyright (c) 2025 Oles Honchar Dnipro National University

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

