SYNTHESIS OF N,N'-(((HYDRAZINE-1,2-DICARBONOTHIOYL)BIS(AZANEDIYL))BIS(2,2,2-TRICHLOROETHANE-1,1-DIYL))CARBOXAMIDES AND THEIR CYCLISATION INTO N,N'-(((1,3,4-THIADIAZOLE-2,5-DIYL)BIS(AZANEDIYL))BIS(2,2,2-TRICHLOROETHANE-1,1-DIYL))CARBOXAMIDES
DOI:
https://doi.org/10.15421/jchemtech.v33i4.338791Keywords:
synthesis, 1,3,4-thiadiazole, oxidative dehydrosulfuration, dithiobiurea, carboxamideAbstract
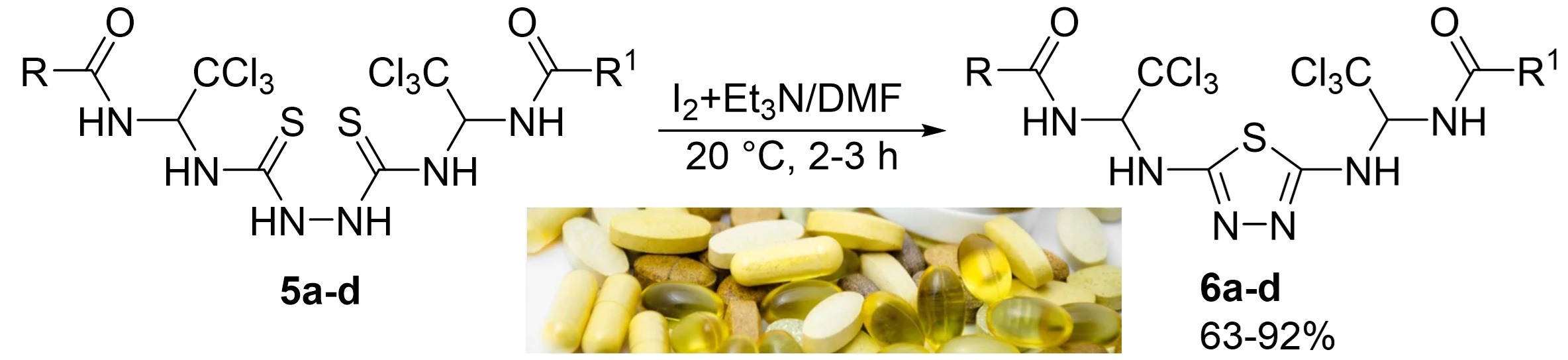
1,3,4-Thiadiazole derivatives are widely used in science and technology as biologically active compounds, components of polymer and rubber compositions, dyes and varnishes, catalysts, as well as materials for microelectronics and nanotechnology. This work presents the synthesis of new bis-amidoalkylated derivatives of 2,5-diamino-1,3,4-thiadiazole. The preparation of these compounds is based on the reaction of oxidative dehydrosulfonation of N,N'-(((hydrazine-1,2-dicarbonothioyl)bis(azanediyl))bis(2,2,2-trichloroethane-1,1-diyl))carboxamides using a mixture of iodine and triethylamine in DMF. The reaction was carried out at room temperature for two hours. This method yields target products in the range of 63–92 %. The advantage of the method is the absence of the need for expensive or hard-to-find reagents. NMR ¹H and ¹³C spectroscopy confirmed the structure of the synthesized compounds. The 1H NMR spectra of synthesized 1,3,4-thiadiazoles are distinguished by the presence of doublet signals corresponding to two NH protons observed in the 9.53–6.69 ppm range, along with a doublet of doublets assigned to the CH proton of the alkylamide fragment appearing at 6.77–6.69 ppm. The 13C NMR spectra exhibit characteristic resonances of the C=O carbon at 168.8–164.7 ppm and the C=N carbon of the thiadiazole ring at 158.9–158.6 ppm. Furthermore, characteristics signals attributed to the CCl3_ moiety and the CH carbon of the alkylamide fragment are observed at 101.5–101.2 and 70.0–69.4 ppm respectively.
References
Hassan, A.A., El-Sheref, E.M. (2010). Chemistry and heterocyclization of dithiobiurea and thioureidoalkylthiourea. Journal of Heterocyclic Chemistry, 47(4), 764–784. https://doi.org/10.1002/jhet.406
Rastogi, R.B., Singh, K., Jaiswal, V. (2014). Synthesis of Triphenyltin (IV) and Dibutyltin (IV) Complexes of 1-Aryl-2,5-dithiohydrazodicarbonamides and Their Characterization. Journal of Applied Chemistry, 2014, 1–5. https://doi.org/10.1155/2014/529764
Matesanz, A.I., Hernández, C., Perles, J., Souza, P. (2016). Synthesis and crystal structure of a novel ruthenium(II) complex with in situ generated dithiobiurea ligand. Journal of Organometallic Chemistry, 804, 13–17. https://doi.org/10.1016/j.jorganchem.2015.12.035
Szécsényi, K.M., Leovac, V.M., Evans, I.R. (2006). Synthesis and characterisation of a novel polymeric Cd complex, catena-(μ-thio)[bis(N-phenylthiourea] bis(dimethylsulphoxide)dichlorocadmium(II). Journal of Coordination Chemistry, 59(5), 523–530. https://doi.org/10.1080/00958970500171240
Prasad, S., Bhattacharya, A., Verma, V.K., Jayanti, S., Rupainwar, D.C. (1992). Synthetic and biocidal studies on the complexes of 1-aryl-2,5-dithiohydrazodicarbonamide with Co(II), Cu(II), and Zn(II). Synthesis and Reactivity in Inorganic and Metal-Organic Chemistry, 22(5), 489–507. https://doi.org/10.1080/15533179208020225
Prasad, K.S., Kumar, L.S., Prasad, M., Revanasiddappa, H.D. (2010). Novel organotin(IV)-Schiff base complexes: synthesis, characterization, antimicrobial activity, and DNA interaction studies. Bioinorganic Chemistry and Applications, 2010, 1–9. https://doi.org/10.1155/2013/502713
Herrero, J.M., Fabra, D., Matesanz, A.I., Hernández, C., Sánchez-Pérez, I., Quiroga, A.G. (2023). Dithiobiureas Palladium(II) complexes' studies: From their synthesis to their biological action. Journal of Inorganic Biochemistry, 246, 112261. https://doi.org/10.1016/j.jinorgbio.2023.112261
Singh, M.M., Rastogi, R.B., Upadhyay, B.N., Yadav, M. (2003). Thiosemicarbazide, phenyl isothiocyanate and their condensation product as corrosion inhibitors of copper in aqueous chloride solutions. Materials Chemistry and Physics, 80(1), 283–293. https://doi.org/10.1016/S0254-0584(02)00513-8
Rastogi, R.B., Singh, M.M., Yadav, M. (2004). Inhibition of corrosion of mild steel by 1-aryl-2,5-dithiohydrazodicarbonamides and their molybdenum and tungsten complexes in 0.1 N sulphuric acid. Bulletin of Electrochemistry, 20, 19–24.
Rastogi, R.B., Yadav, M., Bhattacharya, A. (2002). Application of molybdenum complexes of 1-aryl-2,5-dithiohydrazodicarbonamides as extreme pressure lubricant additives. Wear, 252(9–10), 686–692. https://doi.org/10.1016/S0043-1648(01)00878-X
Firdausiah, S., Hasbullah, S.A., Yamin, B.M. (2018). Synthesis, structural elucidation and antioxidant study of Ortho-substituted N,N’-bis(benzamidothiocarbonyl)hydrazine derivatives. Journal of Physics: Conference Series, 979, 012010. https://doi.org/10.1088/1742-6596/979/1/012010
Odame, F., Hosten, E., Krause, J., Isaacs, M., Hoppe, H., Khanye, S.D., Sayed, Y., Frost, C., Lobb, K., Tshentu, Z. (2020). Synthesis, Characterization and Biological Activity of Some Dithiourea Derivatives. Acta Chimica Slovenica, 67(3), 764–777. https://doi.org/10.17344/acsi.2019.5689
Hunton, P., Sykes, A.H. (1964). The Use of an Anti-Fertility Compound to Delay Sexual Maturity in the Fowl. Poultry Science, 43(6), 1609–1610. https://doi.org/10.3382/ps.0431609
Singh, M.S., Joy, K.P. (2000). Methallibure inhibition of testicular and seminal vesicle activity in catfish, Clarias batrachus (Linn.): a study correlating changes in serum sex steroid profiles. Acta Biologica Hungarica, 51(1), 45–53. https://doi.org/10.1007/BF03542964
Hu, Y., Li, C.Y., Wang, X.M., Yang, Y.H., Zhu, H.L. (2014). 1,3,4-Thiadiazole: synthesis, reactions, and applications in medicinal, agricultural, and materials chemistry. Chemical Reviews, 114(10), 5572–5610. https://doi.org/10.1021/cr400131u
Dawood, K.M. (2019). Bis-thiourea Derivatives and Their Utility in Synthesis of Mono-heterocyclic, Bis-heterocyclic, and Fused Heterocyclic Systems. Journal of Heterocyclic Chemistry, 56(6), 1701–1721. https://doi.org/10.1002/jhet.3540
Adediji, J.F., Adebayo, M.A., Ajayi, Y.O., Yusuf, L.A. (2012). Novel mixed ligand of 2,5-diamino-1,3,4-thiadiazole schiff base incorporating benzoic acid: Synthesis and antimicrobial activity. Journal of Chemical and Pharmaceutical Research, 4(3), 1501–1504.
Yella, R., Khatun, N., Rout, S.K., Patel, B.K. (2011). Tandem regioselective synthesis of tetrazoles and related heterocycles using iodine. Organic & Biomolecular Chemistry, 9(9), 3235–3245. https://doi.org/10.1039/C0OB01007C
Chaudhari, P.S., Pathare, S.P., Akamanchi, K.G. (2012). o-Iodoxybenzoic Acid Mediated Oxidative Desulfurization Initiated Domino Reactions for Synthesis of Azoles. The Journal of Organic Chemistry, 77(8), 3716–3723. https://doi.org/10.1021/jo2025509
Guin, S., Rout, S.K., Gogoi, A., Nandi, S., Ghara, K.K., Patel, B.K. (2012). Desulfurization Strategy in the Construction of Azoles Possessing Additional Nitrogen, Oxygen or Sulfur using a Copper(I) Catalyst. Advanced Synthesis & Catalysis, 354(14–15), 2757–2770. https://doi.org/10.1002/adsc.201200408
Patel, K.N., Jadhav, N.C., Jagadhane, P.B., Telvekar, V.N. (2012). A Novel Strategy for the Construction of Azole Heterocycles via an Oxidative Desulfurization Approach Using Iodobenzene and Oxone®. Synlett, 23(13), 1970–1972. https://doi.org/10.1055/s-0031-1290439
Halimehjani, A.Z., Ashouri, A., Marjani, K. (2012). Dithiocarbamates as an Efficient Intermediate for the Synthesis of Symmetrical Substituted 2,5-Diamino-1,3,4-thiadiazoles in Water. Journal of Heterocyclic Chemistry, 49(4), 939–942. https://doi.org/10.1002/jhet.871
Lin, Q., Zhang, Y.-M., Li, M.-L., Wei, T.-B. (2012). Novel and Efficient Cyclization Procedure for the Synthesis of 2,5-Disubstituted-1,3,4-thiadiazoles Without Using Any Ring-Closing Reagents. Synthetic Communications, 42(22), 3251–3260. https://doi.org/10.1080/00397911.2010.548891
Pavlova, V.V., Zadorozhnii, P.V., Ryabitsky, A.B., Kiselev, V.V., Kharchenko, A.V. (2024). Synthesis and spectral characteristics of N-(2,2,2-trichloro-1-((5-(R-amino)-1,3,4-thiadiazol-2-yl)amino)ethyl)carboxamides. Synthetic Communications, 54(23), 2076–2087. https://doi.org/10.1080/00397911.2024.2422472
Kumar, D., Aggarwal, N., Kumar, V., Chopra, H., Marwaha, R.K., Sharma, R. (2024). Emerging Synthetic Strategies and Pharmacological Insights of 1,3,4-Thiadiazole Derivatives: A Comprehensive Review. Future Medicinal Chemistry, 16, 563–581. https://doi.org/10.4155/fmc-2023-0203
Davinder, K., Harsh, K., Virender, K., Aakash, D., Aastha, S., Minakshi, G.M., Rakesh, M. (2023). Mechanism-Based Approaches of 1,3,4 Thiadiazole Scaffolds as Potent Enzyme Inhibitors for Cytotoxicity and Antiviral Activity. Medicinal Drug Discovery, 17, 100150. https://doi.org/10.1016/j.medidd.2022.100150
Long, K., Boyce, M., Lin, H., Yuan, J., Ma, D. (2005). Structure–activity relationship studies of salubrinal lead to its active biotinylated derivative. Bioorganic & Medicinal Chemistry Letters, 15(17), 3849–3852. https://doi.org/10.1016/j.bmcl.2005.05.120
Zadorozhnii, P.V., Pokotylo, I.O., Kiselev, V.V., Okhtina, O.V., Kharchenko, A.V. (2019). Molecular docking studies of salubrinal and its analogs as inhibitors of the GADD34:PP1 enzyme. ADMET and DMPK, 7(2), 140–150. https://doi.org/10.5599/admet.632
Zadorozhnii, P.V., Kiselev, V.V., Kharchenko, A.V. (2022). In Silico ADME Profiling of Salubrinal and Its Analogues. Future Pharmacology, 2(2), 160–197. https://doi.org/10.3390/futurepharmacol2020013
Schraufstätter, E., Gönnert, R. (1962). Alkyliden- und Aryliden-bis-chloracetamide, eine neue Gruppe gegen Bilharziose wirksamer Verbindungen. Zeitschrift für Naturforschung B, 17(8), 505–516. https://doi.org/10.1515/znb-1962-0804
Zadorozhnii, P.V., Kiselev, V.V., Kharchenko, A.V. (2020). In silico toxicity evaluation of Salubrinal and its analogues. European Journal of Pharmaceutical Sciences, 155, 105538. https://doi.org/10.1016/j.ejps.2020.105538
Drach, B.S., Brovarets, V.S., Smolii, O.B. (1992). [Syntheses of Nitrogen-Containing Heterocyclic Compounds based on Amidoalkylating Agents], Naukova Dumka, Kiev. (in Russian)
Brovarets, V.S., Zyabrev, V.S. (2012). [Synthesis of nitrogen heterocycles based on α-haloalkylamides]. Saarbrücker: LAP LAMBERT Academic Publishing GmbH & Co. KG. (in Russian)
Zadorozhnii, P.V., Pokotylo, I.O., Kiselev, V.V., Kharchenko, A.V., Okhtina, O.V. (2019). Synthesis and spectral characteristics of N-(1-([1,2,4]triazolo[3,4-b][1,3,4]thiadiazol-6-ylamino)-2,2,2-trichloroethyl)carboxamides. Heterocyclic Communications, 25(1), 130–137. https://doi.org/10.1515/hc-2019-0020
Pavlova, V.V., Zadorozhnii, P.V., Kiselev, V.V., Kharchenko, A.V. (2024). Synthesis, spectral characteristics and molecular structure of N-(2,2,2-trichloro-1-(hydrazinecarbothioamido)ethyl)carbox-amides. Chemical Data Collections, 51, 1–9. https://doi.org/10.1016/j.cdc.2024.101137
Downloads
Published
Issue
Section
License
Copyright (c) 2025 Oles Honchar Dnipro National University

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

