Identification of phase structure of plated zinc alloys based on a linear voltammetry in alkaline solutions
DOI:
https://doi.org/10.15421/081616Keywords:
electrolytic zinc alloys, phase analysis, linear voltammetryAbstract
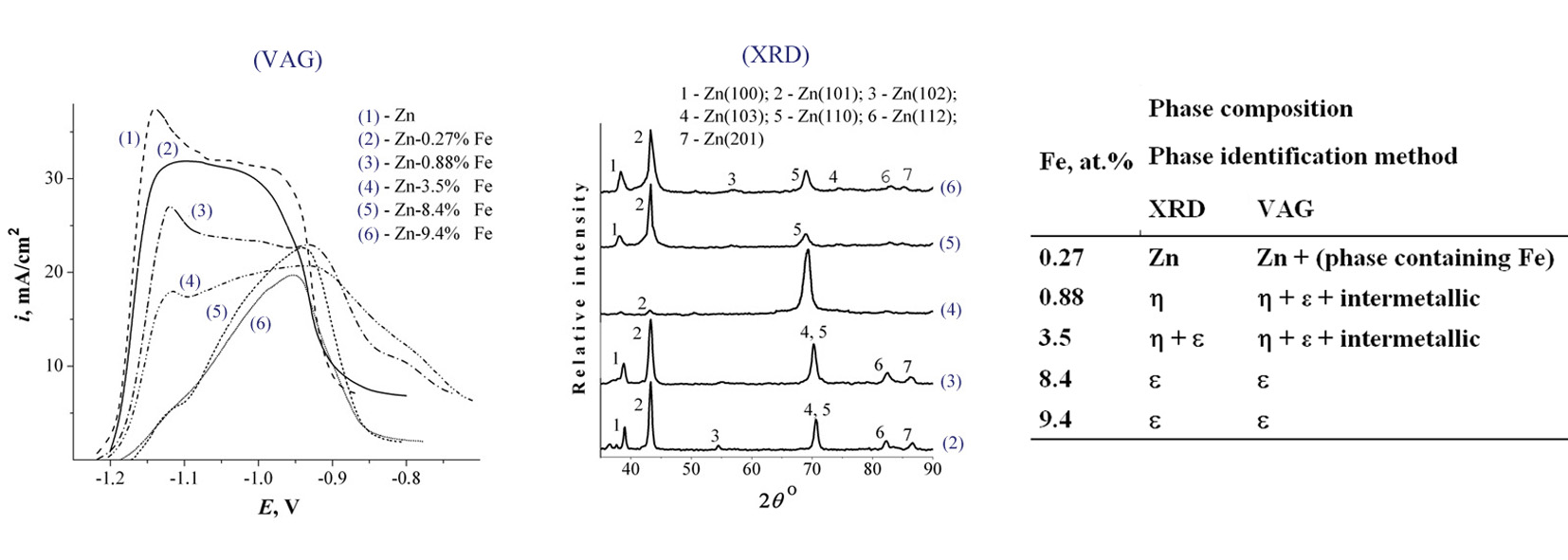
The purpose of research was the development of new and effective technique of electroplatings phase composition analysis by inversion voltammetric methods. As a result the possibility of the phase composition of the plated zinc-based alloys identification using anodic linear voltammetry in alkaline solutions was shown. The phase composition Zn–(0.27–9.4)% Fe alloy electroplated from alkaline zincate solutions was defined based on voltammetry data. As part of the Zn–Fe alloys the phase of hexagonal structure was found which is absent in the equilibrium phase diagram. The ratio of hexagonal crystal lattice axes (c/a) and the electron concentration (e/a) for this phase are significantly different from the corresponding values for the primary solid solution η. From the analysis of c/a and e/a values of investigated Zn–Fe alloy the defined phase was identified as a solid solution phase type ε. It also was shown that anodic linear voltammetry accomplished in alkaline solutions is more sensitive to the identification of the phase composition of zinc alloys than the traditional X-ray method and stripping voltammetry.References
Conrad, H. A., McGuire, M. R., Zhou, T., Coskun, M. I., & Golden, T. D. (2015). Improved corrosion resistant properties of electrochemically deposited zinc-nickel alloys utilizing a borate electrolytic alkaline solution. Surf. Coat. Technol., 272, 50–57. doi:10.1016/j.surfcoat.2015.04.025 CrossRef
Feng, Z., Li, Q., Zhang, J., Yang, P., Song, H., & An, M. (2015). Electrodeposition of nanocrystalline Zn–Ni coatings with single gamma phase from an alkaline bath. Surf. Coat. Technol., 270, 47–56. doi:10.1016/j.surfcoat.2015.03.020 CrossRef
Nakano, H., Arakawa, S., Oue, S., & Kobayashi, S. (2015). Electrodeposition Behavior of Zn–Fe Alloy from Zincate Solution Containing Triethanolamine. Mater. Trans., 56(10), 1664–1669. doi:10.2320/jinstmet.J2014054 CrossRef
Fashu, S., Gu, C. D., Zhang, J. L., Huang, M. L., Wang, X. L., & Tu, J. P. (2015). Effect of EDTA and NH4Cl additives on electrodeposition of Zn–Ni films from choline chloride-based ionic liquid. Trans. Nonferrous Met. Soc. China, 25, 2054−2064. doi:10.1016/S1003-6326(15)63815-8 CrossRef
Tarng, M. L., & Wehner, G. K. (1971). Auger-spectroscopy for structure analysis of coatings. J. Appl. Phys., 42, 2449–2452.
Swathirajan, S. (1986). Potentiodynamic and Galvanostatic Stripping Methods for Characterization of Alloy Electrodeposition Process and Product. J. Electrochem. Soc., 133(4), 671–680. doi:10.1149/1.2108652 CrossRef
Assaf, F. H., Abou-Krisha, M. M., Alduaij, O. K., El-Seidy, A. M. A., & Eissa, A. A. (2015). The Effect Manganese Concentration on the Corrosion Resistance and Physical Properties of Zn-Ni-Mn Alloy Films Produced by Electrodeposition. Int. J. Electrochem. Sci., 10, 6273–6287. Retrieved from electrochemsci.org
Vydra, F., Stulik, K., & Julakova, E. (1978). Electrochemical stripping analysis. New York, Toronto, USA: Halsed Press.
Braynina, Kh. Z., Neyman, Ye. Ya., & Slepushkin, V. V. (1988). [Inversion Electroanalytical Methods]. Мoskow, USSR: Khimiya (in Russian).
Marczenko, Z. (1986). Separation and Spectrophotometric Determination of Elements. Chichester, UK: Ellis Horwood Limited.
Petrenko, L. V. (2016). [The kinetics of anodic dissolution and passivation of zinc alloyed coatings] (Unpublished doctoral dissertation). Oles Honchar Dnipropetrovsk National University, Dnipropetrovsk, Ukraine (in Ukrainian). Retrieved from udhtu.com.ua
Pearson, W. (1977). [Crystal Chemistry and Physics of Metals and Alloys]. Moscow, USSR: Mir (in Russian).
Barrett, C. S., & Massalski, T. B. (1980). Structure of metals. Oxford, UK: Pergamon Press.
Kondo, K., Hinotani, S., & Ohmori, Y. (1988). Crystal structure and morphology of electrodeposited zinc-iron binary alloys. J. Appl. Electrochem., 18(1), 154–161. doi:10.1007/BF01016220 CrossRef
Fujieda, T., Nsganawa, A., Toyota, M., Higuchi, S., & Takahashi, S. (1992). Structure of Electrodeposited Zn-Fe Alloy in the Zinc Rich Region. ISIJ International., 32(9), 1044–1046. Retrieved from jstage
Kryshtop, Yu. G. (2011). [Kinetic and thermodynamic peculiarities of the initial stages of zinc electrocrystallization] (Unpublished doctoral dissertation). Oles Honchar Dnipropetrovsk National University, Dnipropetrovsk, Ukraine (in Russian).
Downloads
Published
Issue
Section
License
Copyright (c) 2016 Oles Honchar Dnipropetrovsk National University

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

