LUMINESCENT PROPERTIES OF Mn4+-DOPED α-Al2O3 OBTAINED BY COMBUSTION METHOD
DOI:
https://doi.org/10.15421/jchemtech.v30i4.259866Keywords:
aluminum oxide, combustion synthesis, Mn4 , luminescence, Racah parameters, luminescence compositesAbstract
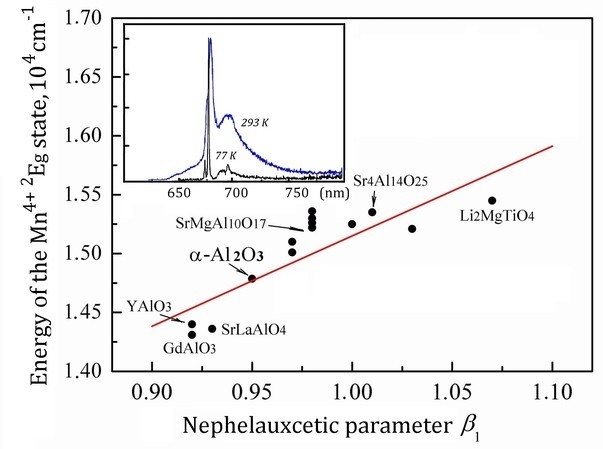
The Mn4+-doped α-Al2O3 with particle size of 70–600 nm was obtained by combustion method. Based on the results of luminescence measurements, the crystal-field strength (Dq) and Racah parameters (B, C) for Mn4+ in α-Al2O3 were determined using a pure electronic transition approach. The obtained values of Dq (1898 cm-1) and nephelauxetic parameter β1 (0.95) for Mn4+ ions in α-Al2O3 are consistent with those of Mn4+ in other oxide compounds. In particular, the Dq/B value of 2.77 indicates a strong crystal field environment of the Mn4+ ions in the α-Al2O3 lattice. A comparison with literature data for Cr3+ in α-Al2O3 was also carried out. The composites of general formula α-Al2O3 : Mn4+, Mg2+/Gd3Al5O12:Ce3+ had been also obtained. It is shown that these materials demonstrate the intense broadband emission with maxima at about 585 and 678 nm. The broad band with a maximum at 585 nm is caused by the 5d→4f transitions of Ce3+ ions in Gd3Al5O12, whereas the band at 678 nm is caused by the Mn4+ 2Eg→4A2g transitions in α-Al2O3. The emission color changes from yellow to deep red with increasing content of α-Al2O3:Mn4+, Mg2+, and the luminescence quantum efficiency of the composites was found as high as 0.60.
References
Zhou, Q., Dolgov, L., Srivastava, A., M., Zhou, L., Wang, Z., Shi, J., Dramićanin, M. D., Brik, M. G., Wu, M. (2018). Mn2+ and Mn4+ red phosphors: synthesis, luminescence and applications in WLEDs. A review. J. Mater. Chem. C, 6(11), 2652–2671.
https://doi.org/10.1039/C8TC00251G.
Adachi, S. (2018). Photoluminescence properties of Mn4+-activated oxide phosphors for use in white-LED applications: A review. J. Lumin., 202, 263–281. https://doi.org/10.1016/j.jlumin.2018.05.053.
Li, X., Chen, Z., Wang, B., Liang, R., Li, Y., Kang, L., Liu, P. (2019). Effects of impurity doping on the luminescence performance of Mn4+-doped aluminates with the magnetoplumbite-type structure for plant cultivation. Materials, 12, 86.
https://doi.org/10.3390/ma12010086.
Sijbom, H. F., Verstraete, R., Joos, J. J., Poelman D., Smet, P. F. (2017). K2SiF6:Mn4+ as a red phosphor for displays and warm-white LEDs: a review of properties and perspectives. Opt. Mater. Express, 7(9), 3332–3365. https://doi.org/10.1364/OME.7.003332.
Brik, M. G., Camardello, S. J., Srivastava, A. M. (2015). Influence of covalency on the Mn4+ 2Eg→4A2g emission energy in crystals. ECS J. Solid State Sci. Technol., 4(3), R39–R43. https://doi.org/10.1149/2.0031503jss.
Brik, M. G., Camardello, S. J., Srivastava, A. M., Avram, N. M., Suchocki, A. (2016). Spin-forbidden transitions in the spectra of transition metal ions and nephelauxetic effect. ECS J. Solid State Sci. Technol., 5(1), R3067–R3077. https://doi.org/10.1149/2.0091601jss.
Adachi, S. (2020). Mn4+ and Cr3+ ions in red and deep red-emitting phosphors: spectral analysis and Racah parameter determination. J. Lumin., 223, 117217/1–8. https://doi.org/10.1016/j.jlumin.2020.117217.
Tian, C., Lin, H., Zhang, D., Zhang, P., Hong, R., Han, Z., Qian, X., Zou, J. (2019). Mn4+ activated Al2O3 red-emitting ceramic phosphor with excellent thermal conductivity. Opt. Express, 27(22), 32666–32678. https://doi.org/10.1364/OE.27.032666.
Mykhaylyk, V. B., Kraus, H., Bulyk, L.-I., Lutsyuk, I., Hreb, V., Vasylechko, L., Zhydachevskyy, Y., Wagnera, A., Suchocki, A. (2021). Al2O3 co-doped with Cr3+ and Mn4+, a dual emitter probe for multimodal non-contact luminescence thermometry. Dalton Trans., 50, 4820–14831. https://doi.org/10.1039/D1DT02836G.
Dotsenko, V. P., Berezovskaya, I. V., Voloshinovskii, A. S., Zadneprovskii, B. I., Efryushina, N. P. Luminescence properties and electronic structure of Ce3+-doped gadolinium aluminum garnet. (2015). Mater. Res. Bull., 64, 151–155.
https://doi.org/10.1016/j.materresbull.2014.12.056.
Berezovskaya, I. V., Voloshinovskii, A. S., Khapko, Z. A., Khomenko, O. V., Efryushina, N. P., Dotsenko, V. P. (2021). Temperature quenching of the Ce3+ emission in gadolinium aluminum garnet Gd3Al5O12. Func. Mater., 28(1), 6–13. https://doi.org/10.15407/fm28.01.6.
Jain, A., Koyani, R., Muñoz, C., Sengar, P., Conteras, O. E., Juárez, P., Hirata G. A. (2018). Magnetic-luminescent cerium-doped gadolinium aluminum garnet nanoparticles for simultaneous imaging and photodynamic therapy of cancer cells. J. Colloid Interface Sci., 526, 220–229.
https://doi.org/10.1016/j.jcis.2018.04.100.
Zolotko, A. N., Poletaev, N. I., Vovchuk, Ya. I. (2015). Gas-disperse synthesis of metal oxide particles. Comb. Expl. Shock Waves, 51(2), 252–268.
https://doi.org/10.1134/S0010508215020094.
Dotsenko, V. P., Berezovskaya, I. V., Zubar, E. V., Efryushina, N. P., Poletaev, N. I., Doroshenko, Yu. A., Stryganyuk, G. B., Voloshinovskii, A. S. (2013). Synthesis and luminescent study of Ce3+-doped terbium-yttrium aluminum garnet. J. Alloys Compd., 550, 159–163. https://doi.org/10.1016/j.jallcom.2012.09.053.
Berezovskaya, I. V., Poletaev, N. I., Khlebnikova, M. E., Zatovsky, I. V., Bychkov, K. L., Efryushina, N. P., Khomenko, E. V., Dotsenko, V. P. (2016). Luminescence study of nanosized Al2O3:Tb3+ obtained by gas-dispersed synthesis. Methods Appl. Fluoresce., 4, 034011/1–8. https://doi.org/10.1088/2050-6120/4/3/034011
Berezovskaya, I. V., Khomenko, E. V., Poletaev, N. I., Khlebnikova, M. E., Stoyanova, I. V., Efryushina, N. P., Dotsenko, V. P. (2018). Oxidation states and microstructure of manganese impurity centers in nanosized Al2O3 obtained by combustion method. Func. Mater., 25, 490–495.
https://doi.org/10.15407/fm25.03.490.
Feofilov, S. P., Kulinkin, A. B., Kutsenko, A. B., Zakharchenya, R. I. (1998). Selective laser spectroscopy of RE3+ and Mn4+ in sol-gel technique produced Al2O3. J. Lumin., 76 & 77, 217–220.
https://doi.org/10.1016/S0022-2313(97)00204-4.
Baronskiy, M., Rastorguev, A., Zhuzhgov, A., Kostyukov, A., Krivoruchko, O., Snytnikov, V. (2016). Photoluminescence and Raman spectroscopy studies of low-temperature γ-Al2O3 phases synthesized from different precursors. Opt. Mater., 53, 87–93. https://doi.org/10.1016/j.optmat.2016.01.029.
Geschwind, S., Kisliuk, P., Klein, M. P., Remeika, J. P., Wood D. L. (1962). Sharp-line fluorescence, electron paramagnetic resonance, and thermoluminescence of Mn4+ in α-Al2O3. Phys. Rev., 126(5), 1684–1686. https://doi.org/10.1103/PhysRev.126.1684.
Van Die, A., van der Weg, W. F., Leenaers, A. C. H. I., Blasse, G. (1987). A search for luminescence of the trivalent manganese ion in solid aluminates. Mat. Res. Bull., 22, 781–788. https://doi.org/10.1016/0025-5408(87)90032-8.
Kück, S., Hartung, S., Hurling, S., Petermann, K., Huber, G. (1998). Optical transitions in Mn3+-doped garnets. Phys. Rev. B, 57(4), 2203–2216. https://doi.org/10.1103/PhysRevB.57.2203.
Noginov, M. A., Loutts, G. B., Warren M. (1999). Spectroscopic studies of Mn3+ and Mn2+ ions in YAlO3. J. Opt. Soc. Am. B, 16(3), 475–483. https://doi.org/10.1364/JOSAB.16.000475.
Srivastava, A. M., Brik, M. G. (2017). The nature of Mn4+ luminescence in the orthorhombic perovskite, GdAlO3. Opt. Mater., 63, 207–212. https://doi.org/10.1016/j.optmat.2016.06.032.
Adachi, S. (2020). Crystal-field and Racah parameters of Mn4+ ion in red and deep red-emitting phosphors: Fluoride versus oxide phosphor. J. Lumin., 218, 116829/1–8. https://doi.org/10.1016/j.jlumin.2019.116829.
Dotsenko, V. P., Berezovskaya, I. V., Poletaev N. I., Khlebnikova M. E., Zatovsky I. V., Bychkov K. L., Khomenko, E. V., Efryushina, N. P. (2021). Combustion synthesis and nontrivial luminescence properties of nanosized δ*-Al2O3 doped with Cr3+ ions. Solid State Sci., 119, 106704/1–8. https://doi.org/10.1016/j.solidstatesciences.2021.106704.
Adachi, S. (2021). Luminescence spectroscopy of Cr3+ in Al2O3 polymorphs. Opt. Mater., 114, 111000/1–8. https://doi.org/10.1016/j.optmat.2021.111000.
Li, K., Xue, D. (2006). Estimation of electronegativity values of elements in different valence states. J. Phys. Chem. A, 110, 11332–11337. https://doi.org/10.1021/jp062886k.
Xia Z., Meijerink A. (2017). Ce3+-Doped garnet phosphors: composition modification, luminescence properties and applications. Chem. Soc. Rev., 46, 275–299. https://doi.org/10.1039/C6CS00551A
Li X., Chen J., Liu Z., Deng Z., Huang Q., Huang J., Guo W. (2022). (Ce, Gd):YAG-Al2O3 composite ceramics for high-brightness yellow light-emitting diode applications. J. Eur. Ceram. Soc., 42, 1121–1131. https://doi.org/10.1016/j.jeurceramsoc.2021.11.027
Katelnikovas, A., Bettentrup, H., Uhlich, D., Sakirzanovas, S., Justel, T., Kareiva, A. (2009). Synthesis and optical properties of Ce3+-doped Y3Mg2AlSi2O12. J. Lumin., 129, 1356–1361. https://doi.org/10.1016/j.jlumin.2009.07.006
Downloads
Published
Issue
Section
License
Copyright (c) 2022 Oles Honchar Dnipro National University

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

