FEATURES OF THE STRUCTURE OF COPPER-CONTAINING COMPOSITES BASED ON Cu+ MALEATE COMPLEXES
DOI:
https://doi.org/10.15421/jchemtech.v31i4.290194Keywords:
Cu maleate complexes, copper-containing composites, quantum chemical modeling., X-ray phase analysisAbstract
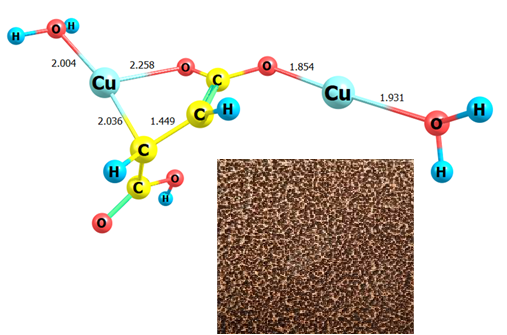
Quantum chemical modeling (Gaussian 09, AIM2000, Chemcraft 1.8) of the interaction of copper atoms with acidic maleate complexes Cu+ [Cu(НМ)(Н2О)] made it possible to identify two types of thermodynamically stable binuclear π-complexes of the general composition [Cu2(НМ)(H2O)2]. Type A is characterized by a framework structure in which both Cu+ ions and Cu0 atoms form π-bonds with sp2-hybridized carbon atoms of the vinyl fragment of the maleate ion within separate six-membered cycles (-Cu-С-С=О-Н-О-). Type B is a linear σ-connection of a hydrated copper atom with the carboxyl oxygen of the maleate ion. The closeness of the formation energies of molecules A and B (−114.39 kJ/mol and −127.84 kJ/mol, respectively) indicates a high probability of their simultaneous formation during the synthesis of the composite {Cu(НМ)+Cu}. X-ray diffraction analysis of composite samples confirmed that there is no metallic copper phase in it, but there is a phase of a new substance – products of the interaction of Cu0 atoms with π-complexes [Cu(НМ)(Н2О)]. The analysis of the obtained results of our theoretical and experimental research indicates that during the synthesis of copper-containing composites {Cu(НМ)+Cu} by partial chemical reduction of maleate complexes of Cu+, a mixture of mononuclear π-complexes [Cu(HM)(H2O)] with various binuclear π-complexes [Сu2(НМ)(Н2О)2] is formed.
References
Guo Z, Sadler P. J. (1999). Metals in medicine. Angewandte Chemie International Edition 1999; 38 (11): 1512–1531. http://doi: 10.1002/(SICI)1521-3773(19990601)38:11<1512::AID-ANIE1512>3.0.CO;2-Y
Hossain, M. S., Zakaria, C. M., Kudrat-E-Zahan, M. (2018). Metal complexes as potential antimicrobial agent: a review. American Journal of Heterocyclic Chemistry, 4(1), 1. http://dx.doi.org/10.11648/j.ajhc.20180401.11.
Aldabaldetrecu, M., Tamayo, L., Alarcon, R., Walter, M., Salas-Huenuleo, E., Kogan, M. J., Guerrero, J., Paez, M., Azócar, M. I. (2018). Stability of Antibacterial Silver Carboxylate Complexes against Staphylococcus epidermidis and Their Cytotoxic Effects. Molecules, 23(7), 1629.http://dx.doi.org/10.3390/molecules23071629.
Irfan, M. I., Amjad, F., Abbas, A., Rehman, M. F., Kanwal, F., Saeed, M., Ullah, S., Lu, C. (2022). Novel Carboxylic Acid-Capped Silver Nanoparticles as Antimicrobial and Colorimetric Sensing Agents. Molecules, 27, 3363. http://dx.doi.org/10.3390/molecules27113363.
Kalhapure, R. S., Akamanchi, K. G., Mocktar, C., Govender, T. (2014). Synthesis and Antibacterial Activity of Silver Nanoparticles Capped with a Carboxylic Acid-terminated Generation 1 Oleodendrimer. Chemistry letters, 43(7), 1110–1112. http://dx.doi.org/10.1246/cl.140151.
Theivasanthi, T., Alagar, M. (2011). Studies of copper nanoparticles effects on microorganism. Annals of Biological Research, 2(3), 368–373.
Sirova, G. O., Makarov, V. O., Mishina, M. M., Avramenko, V. L., Lapshin, V. V., Makarov, V. V. (2019). Copper – nanocopper: chemical and pharmaceutical aspect: monograph. Kharkiv: Planet-Print (in Ukrainian).
Vargalyuk, V. F., Polonskyy, V. А., Stets, O. S., Stets, N. V., Shchukin, A. І. (2014). [Microbiological properties of copper dispersion obtained by cathodic deposition in the presence of acrylic acid]. Bulletin of Dnipropetrovsk University. Series Chemistry, 22(2), 47–51 (in Ukrainian). http://dx.doi.org/10.15421/081420.
Vargaluyk, V. F., Polonskyy, V. A., Sklyar, T. V., Stets, N. V., Lahuta, O. V. (2023). Pysico-chemical and bactericidal properties of copper containing composites based on maleinate complexes Cu+. Journal of Chemistry and Technologies, 31(2), 208–215. https://doi.org/10.15421/jchemtech.v31i2.275070
Aliakbar Dehno Khalaji, Moslem Emami, Negin Mohammadi (2021). Antibacterial Activity of Copper (II) Complexes of Maleic Acid: Thermal Studies, and New Precursors for Preparation of CuO. Journal of Medicinal and Chemical Sciences, 4, 626–634. https://doi.org/10.26655/JMCHEMSCI.2021.6.11
Kurasova, Y. D., Vargaluyk, V. F., Polonskyy, V. A. (2022). Quantum chemical modeling of aquachlorocomplexes of Cu+ with acrylic, maleik and fumaric acids Journal of Chemistry and Technologies, 30(4), 530–536. https://doi.org/10.15421/jchemtech.v30i4.263280
Vargalyuk, V. F., Osokin, Y. S., Polonskyy, V. A., Glushkov, V. N. (2019). Features of (dπ-pπ)-binding of Cu(I) ions with acrylic, maleic and fumaric acids in aqueous solution. Journal of Chemistry and Technologies, 27(2), 148–157. https://doi.org/10.15421/081916/
Kamau, P., Jordan, R. B. (2002). Formation Constants of Copper (I)− Olefin Complexes in Aqueous Solution. Inorganic chemistry, 41(4), 884–891. doi: 10.1021/ic010872h
Navon, N., Masarwa, A., Cohen, H., Meyerstein, D. (1997). pH dependence of the stability constants of copper (I) complexes with fumaric and maleic acids in aqueous solutions. Inorganica chimica acta, 261(1), 29–35. https://doi.org/10.1016/S0020-1693(96)05575-2
Orlova, T. D., Katrovtseva, A. V., Bychkova, S. A., Lan, Fam Tkhi. (2011). The thermodynamic characteristics of formation of Copper(II) ion complexes with carboxylic acids in aqueous solutions. Journal of Coordination Chemistry. 85(2), 275–279. http://doi: 10.1134/S0036024411020269
Ardan, B., Kinzhybalo, V., Slyvka, Y., Shyyka, O., Luk’yanov, M., Lis, T., Mys’kiv, M. (2017). Ligand forced dimerization of copper(I)-olefin complexes bearing 1,3,4-thiadiazole core. Acta Cryst, C73, 36–46. https://doi.org/10.1107/S2053229616018751
Goreshnik E. A., Veryasov G., Morozov D.et al. (2016). Solvated copper(I) hexafluorosilicate π-complexes based on [Cu2(amtd)2]2+ (amtd = 2-allylamino-5-methyl-1,3,4-thiadiazole) dimer. J. Organomet. Chem, 810, 1–11. https://doi.org/10.1016/j.jorganchem.2016.03.001
Luk’yanov, M., Slyvka, Yu., Ardan, B., Mys’kiv, M. (2018). Synthesis and crystal structure of the π-complex of cuprum (І) sulfamate З2-(N-alil)-amino-5-metyl-1,3,4-thiadiazole composition |Cu2(C6H10N3S2)2(NH2SO3)2|. Visnyk Lviv. Univ. Ser. Chem., 59(1), 157–163 (in Ukrainian).
Vargalyuk V. F., Osokin, Y. S., Polonskyy, V. A. (2020). Formation of the π-complexes of copper atoms with acrylic, maleic and fumaric acids in aqueous medium. Journal of Chemistry and Technologies, 28(2), 153 160. https://doi.org/10.15421/082016
Vargaluyk, V. F., Polonskyy, V. A., Osokin, Y. S., Lahuta, O. V. (2021). Syntesis of copper composites containing maleic acid. Journal of Chemistry and Technologies, 29(3), 400–409. http://dx.doi.org/10.15421/jchemtech.v29i3.241965.
Altomare A. Corriero, N., Cuocci, C., Falcicchio, A., Moliterni, A., Rizzi R. (2017). Main features of QUALX2.0 software for qualitative phase analysis. Powder Diffraction, 32(1), S129–S134. https://doi.org/10.1017/S0885715617000240.
Frisch, M. J. E. A., Trucks, G. W., Schlegel, H. B., Scuseria, G. E., Robb, M. A., Cheeseman, J. R., Nakatsuji, H. (2009). Gaussian 09, Revision A. 02, Gaussian. Inc., Wallingford, CT, 200(28).
König, F. B., Schönbohm, J., Bayles, D. (2001). AIM2000 – A program to analyze and visualize atoms in molecules. Journal of Computational Chemistry, 22(5), 545–559.
Zhurko, G. A. (2019). Chemcraft-graphical program for visualization of quantum chemistry computations, Version 1.8., from https://chemcraftprog.com.
Vargaljuk, V., Okovytyy, S., Polonskyy V., Kramska O., Shchukin A., Leszczynski J. (2016) Copper crystallization from aqueous solution: initiation and evolution of the polynuclear clusters. Journal of Cluster Science, 28(5), 1–12. doi: 10.1007/s10876-017-1239-4
Zavalij, P. E., Mys'kiv M. G., Gladyshevskij, E. I. (1985). Kristallicheskaja struktura monogidrata kislogo maleata medi(I). Kristallografija, 30, 688–692.
Momma, K., Izumi, F. (2011). VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. Journal of Applied Crystallography, 44(6), 1272–1276. https://doi.org/10.1107/S0021889811038970
Downloads
Published
Issue
Section
License
Copyright (c) 2023 Oles Honchar Dnipro National University

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

