INTERACTION OF LABILE N-ALKOXY-N-CHLORO-N’-ARYLUREAS AND N-ACETOXY-N-ALKOXYUREAS WITH TRIMETHYL PHOSPHITE
DOI:
https://doi.org/10.15421/jchemtech.v33i3.332948Keywords:
N-alkoxy-N-chloro-N’-arylureas; trimethyl phosphite; dimethyl N-alkoxy-N-(N’-arylcarbamoyl)phosphoroamidates; synthesis.Abstract
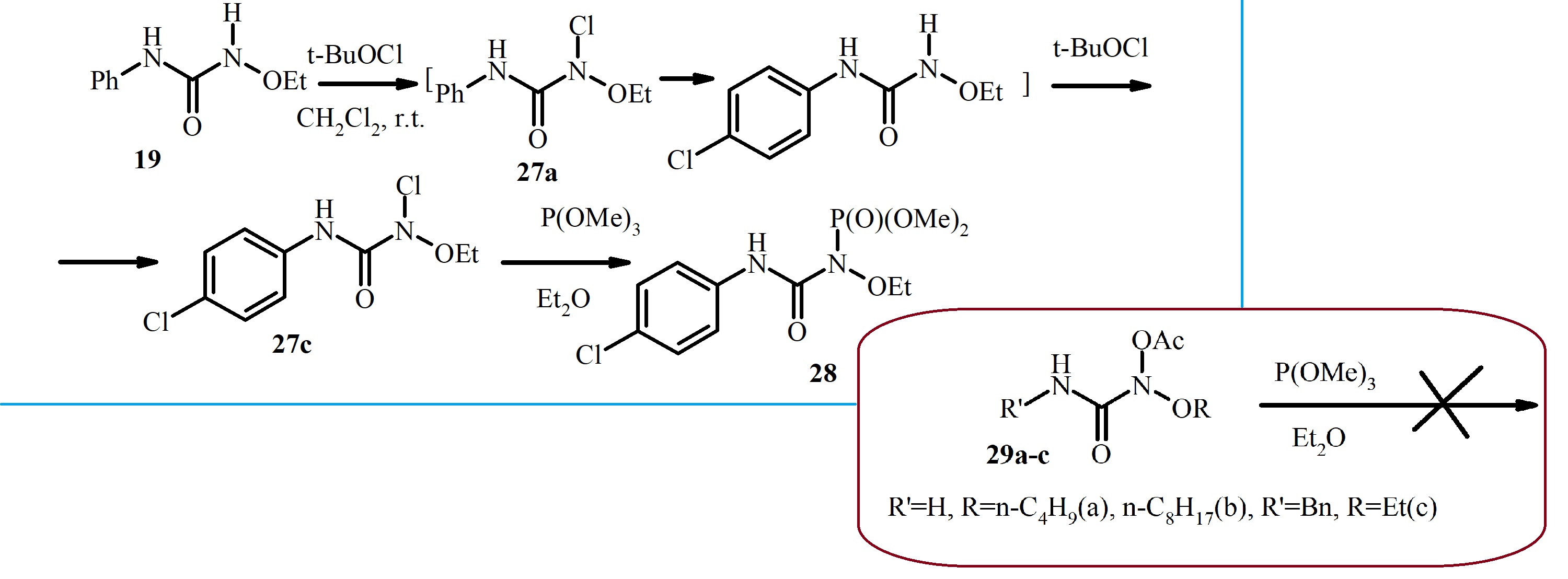
The freshly synthesized N-alkoxy-N-chloro-N’-4-bromophenylureas undergo reaction with trimethyl phosphite in diethyl ether at room temperature yielding respectively dimethyl N-alkoxy-N-(N’-4-bromophenylcarbamoyl)phosphoroamidates with high yields. The unstable N-alkoxy-N-chloro-N’-phenylureas, freshly synthesized at -30°C, interact with trimethyl phosphite in diethyl ether at this low temperature to produce previously unknown dimethyl N-alkoxy-N-(N’-phenylcarbamoyl)phosphoroamidates. This reaction is the first example of the nucleofilic substitution at the nitrogen atom for unstable N-alkoxy-N-chloro-N’-phenylureas. Careful conditions selection and precise control made it possible to pevent premature destruction of the starting N-alkoxy-N-chloro-N’-4-bromophenylureas and N-alkoxy-N-chloro-N’-phenylureas. In contrast, N-acetoxy-N-alkoxyureas do not react with trimethyl phosphite under the same conditions. The structures of the resulting dimethyl N-alkoxy-N-(N’-4-bromophenylcarbamoyl)phosphoroamidates and dimethyl N-alkoxy-N-(N’-phenylcarbamoyl)phosphoroamidates were confirmed by ¹H, ³¹P, and ¹³C NMR spectroscopy, as well as mass spectrometry. A comparative analysis of ¹H, ³¹P and ¹³C NMR spectra of these dimethyl N-alkoxy-N-(N’-arylcarbamoyl)phosphoroamidates with those of dialkyl N-alkoxy-N-(N’-4-nitrophenylcarbamoyl)phosphoroamidates revealed numerous shared features and general structural characteristics of N-alkoxy-N-(N’-arylcarbamoyl)phosphoroamidates.
References
Ghosh, A.K., Brindisi, M. (2019). Urea Derivatives in Modern Drug Discovery and Medicinal Chemistry. J Med Chem. 63(6), 2751–2788. doi: 10.1021/acs.jmedchem.9b01541
Itumoh, E.J., Data, S., Leitao, E.M. (2020). Opening up the Toolbox: Synthesis and Mechanism of Phosporamidates. Molecules. 25(16), 3684. https://doi.org/10.3390/molecules25163684
Shtamburg, V.G., Klots, E.A., Shtamburg, V.V., Anishchenko, A.A., Shishkina, S.V., Mazepa, A.V. (2023). Nucleophilic substitution at nitrogen atom. N-Alkoxy-N-(dimethoxyphosphoryl)ureas, synthesis and structure. J. ,Mol. Structure, 1277, 134865. https://doi.org/10.1016/j.molstruc.2022.134865
Shtamburg V.G., Klots, E.A., Anishchenko, A.A., Shishkina, S.V., Mazepa, A.V., Kravchenko, S.V. (2024). Interaction of N-Alkoxy-N-chloro derivatives of amides, sulfonamides and ureas with trialkyl phosphites. XXVI Ukrainian Conference of Organic Chemistry and Biochemistry, Uzhgorod, 16-20 September 2024, Thesis, D-5. (In Ukrainian)
Shtamburg, V.G., Klots, E.A., Anishchenko, A.A., Shtamburg, V.V., Shishkina, S.V., Mazepa, A.V., Kravchenko, S.V. (2025). Interaction of N-Alkoxy-N-chloro-N’-arylureas with Trialkyl Phosphites as Route to Dialkyl N-Alkoxy-N-(N’-arylcarbamoyl)phosphoramidates. Synthesis. Voprosy khimii i khimicheskoi tecknologii – Issues of Chemistry and Chemical Technology, 2025, (5): in press.
Perronnet, J., Demoute, J.P. (1982). Approach to the 1-methoxy-2-benzimidazolinones. Gazzet. Chim. Ital., 112, 507–511.
Shtamburg, V.G., Tsygankov, A.V., Gerasimenko, M.V., Shishkin, O.V., Zubatyuk, R.I., Mazepa, A.V., Kostyanovsky, R.G. (2011). New approach to N,N-dialkoxy-N'-arylureas and N,N-dialkoxycarbamates. Mendeleev Commun., 21(1), 50−52. https://doi.org/10.1016/j.mencom.2011.01.021
Glover, S.A. (1998). Anomeric Amides – Structure, Properties and Reactivity. Tetrahedron, 54(26), 7229−7272. https://doi.org/10.1016/S0040-4020(98)00197-5
Cavanagh, K.L., Glover, S.A., Price, H.L., Schumacher, R.R. (2009). SN2 Substitution reactions at the Amide Nitrogen in the Anomeric Mutagens, N-Acyloxy-N-alkoxyamides. Aust. J. Chem., 62(7), 700–710. https://doi.org/10.1071/CH09166
Glover, S.A. (2009). N-Heteroatom-substituted hydroxamic esters, in The Chemistry of Hydroxylamines, Oximes and Hydroxamic Acids. Eds Rappoport, Z., Liebman, J. F., John Wiley and Sons, New York. PATAI’S Chemistry of Funcional Groups https://doi.org/10.1002/9780470682531.pat0470
Glover, S.A., Rosser, A.A. (2018). Heteroatom Substitution at Amide Nitrogen – Resonance Reduction and HERON Reactions of Anomeric Amides. Molecules, 23(11), 2834. https://doi.org/10.3390/molecules23112834
Cavanagh, K.L. Glover, S.A., Price, H.L. (2009). Schumacher, SN2 Substitution reactions at the Amide Nitrogen in the Anomeric Mutagens, N-Acyloxy-N-alkoxyamides. Aust. J. Chem., 62(7): 700–710. https://doi.org/10.1071/CH09166
Shtamburg, V.G., Tsygankov, A.V., Shishkin, O.V., Zubatyuk, R.I., Uspensky, B.V., Shtamburg, V.V., Mazepa, A.V., Kostyanovsky, R.G. (2012). The properties and structure of N-chloro-N-methoxy-4-nitrobenzamide. Mendeleev Commun., 22(3) 164–166. https://doi.org/10.1016/j.mencom.2012.05.019.
Digianantonio, K.M., Glover, S.A., Johns, J.P., Rosser, A.A. (2011). Synthesis and termal decomposition of N,N-dialkoxyamides. Org. Biomol. Chem., 9, 4116−4126. https://doi.org./10.1039/C1OB00008J
Glover, S.A., White, J.M., Rosser, A.A., Digianantonio, K.M. (2011). Structure of N,N-Dialkoxyamides: Pyramidal Anomeric Amides with Low Amidicity. J. Org. Chem., 76(23), 9757–9763. https://doi.org./10.1021/jo201856u
Glover, S.A., Rosser, A.A., Taherpour, A., Greatrex, B. (2014). Formation and HERON Reactivity of Cyclic N,N-Dialkoxyamides. Aust. J. Chem., 67(3), 507–520 https://doi.org./10.1071/CH13557
Pu, X., Li, Q., Lu, Z.,Yang, X. (2016). N-Chloro-N-methoxybenzenesulfonamide: A Chlorinating Reagent. Eur. J. Org. Chem., (36), 5937−5940. https://doi.org/10.1002/ejoc.201601226
Wang Yu, Bi C., Kawamata Yu, Grant L.N., Samp L., Richardson P.F., Zhang S., Harper K.C., Palkowitz M.D., Vasilopoulos, A., Collins M.R., Oderinde, M.S., Tyrol, C.C., Chen, D., LaChapelle, E.A., Bailey, J.B., Qiao, J.X., Baran, P.S. (2024). Discovery of N−X anomeric amides as electrophilic halogenation reagents. Nature Chemistry, 16, 1539−1545 https://doi.org/10.1038/s41557-024-01539-4.
Shtamburg, V.G., Tsygankov, A.V., Klots, E.A., Kostyanovsky, R.G. (2004). Acyloxy group exchange in N-acyloxy-N-alkoxyamides. Mendeleev Commun., 14(5) 208–210. https://doi.org/10.1070/MC2004v014n05ABEH001908
Shtamburg, V.G., Shishkin, O.V., Zubatyuk, R.I., Kravchenko, S.V., Tsygankov, A.V., Mazepa, A.V., Klots, E.A., Kostyanovsky, R.G. (2006). N-Chloro-N-alkoxyureas: synthesis, structure and properties. Mendeleev Commun., 16(3) 323–325. https://doi.org/10.1070/MC2006v016n06ABEH002382
Downloads
Published
Issue
Section
License
Copyright (c) 2025 Oles Honchar Dnipro National University

This work is licensed under a Creative Commons Attribution 4.0 International License.
- Authors reserve the right of attribution for the submitted manuscript, while transferring to the Journal the right to publish the article under the Creative Commons Attribution License. This license allows free distribution of the published work under the condition of proper attribution of the original authors and the initial publication source (i.e. the Journal)
- Authors have the right to enter into separate agreements for additional non-exclusive distribution of the work in the form it was published in the Journal (such as publishing the article on the institutional website or as a part of a monograph), provided the original publication in this Journal is properly referenced
- The Journal allows and encourages online publication of the manuscripts (such as on personal web pages), even when such a manuscript is still under editorial consideration, since it allows for a productive scientific discussion and better citation dynamics (see The Effect of Open Access).

